雷贝拉唑对NSAIDs相关小肠损伤大鼠紧密连接蛋白Occludin表达的影响及其机制
高 欣,张振玉,吴海露,胡可伟,姜宗丹,杨小兵,王劲松
南京医科大学附属南京第一医院1.消化科;2.病理科,江苏 南京 210006
雷贝拉唑对NSAIDs相关小肠损伤大鼠紧密连接蛋白Occludin表达的影响及其机制
高 欣1,张振玉1,吴海露1,胡可伟1,姜宗丹1,杨小兵2,王劲松2
南京医科大学附属南京第一医院1.消化科;2.病理科,江苏 南京 210006
目的 探讨雷贝拉唑对非甾体类抗炎药(NSAIDs)相关性小肠损伤大鼠紧密连接蛋白Occludin表达的影响及可能的机制。方法 将36只SD大鼠随机平均分为阴性对照组、双氯酚酸损伤组和雷贝拉唑处理组。采用双氯酚酸7.5 mg/(kg·d)灌胃,连续4 d,制造大鼠NSAIDs相关性小肠损伤模型;而雷贝拉唑处理组在每次造模前0.5 h予以15 mg/(kg·d)雷贝拉唑灌胃处理,连续4 d。处死大鼠进行大体及病理观察小肠损伤情况,采用免疫组织化学和Western blot方法检测小肠组织中Occludin和磷酸化ERK(p-ERK)蛋白表达水平的变化。结果 雷贝拉唑处理组大鼠大体和病理损伤均低于损伤组(P<0.05)。Occludin蛋白在损伤组中表达较对照组明显下降(P<0.05),而在雷贝拉唑处理组中的表达较损伤组上升(P<0.05);与阴性对照组相比,p-ERK蛋白在损伤组中表达上升(P<0.05),在雷贝拉唑处理组中的表达较损伤组下降(P<0.05)。结论 雷贝拉唑对大鼠NSAIDs相关性损伤有保护作用,其机制可能是通过MAPK中的ERK途径,增加小肠上皮组织中Occludin蛋白表达,从而增强肠黏膜屏障功能。
雷贝拉唑;NSAIDs相关性小肠损伤;Occludin蛋白;MAPK/ERK信号通路
非甾体抗炎药(NSAIDs)临床应用日趋广泛,NSAIDs相关性消化道反应也日益受到关注,但长期以来,NSAIDs相关性小肠损伤一直被人们忽视。近年来,随着胶囊内镜和双气囊小肠镜检查的普及,研究已发现NSAIDs相关的小肠损伤在使用NSAIDs患者中发生并不罕见,损伤程度甚至超过了胃损伤[1]。质子泵抑制剂(PPI)对NSAIDs相关性胃黏膜损伤有很好的保护作用,而对NSAIDs相关性小肠损伤的影响则存在一定争议。本实验旨在研究雷贝拉唑对大鼠NSAIDs相关性小肠损伤及其紧密连接蛋白Occludin表达的影响,并探讨其可能的机制。
1 材料与方法
1.1 材料 2个月龄体质量180~200 g的健康雄性SD大鼠30只(由南京医科大学附属南京第一医院动物实验中心提供);雷贝拉唑钠肠溶片(商品名:信卫安,产品批号:110503)由上海信谊药厂有限公司馈赠,双氯酚酸钠双释放肠溶胶囊(商品名:戴芬,产品批号:91184)购自德国Temmler Werke GmbH公司,两种药物均溶于生理盐水中,经超声乳化后制成悬浊液;磷酸化 ERK(p-ERK)、Occludin抗体购自 cell signal公司。
1.2 方法
1.2.1 动物分组:将30只大鼠随机分为3组,每组10只:1组,阴性对照组;2组,双氯酚酸损伤组;3组,雷贝拉唑处理组。
1.2.2 动物造模:除阴性对照组外,其他两组大鼠按照双氯酚酸7.5 mg/kg剂量进行灌胃,1次/d,连续4 d,制造大鼠急性胃黏膜损伤模型。每次造模前0.5 h,雷贝拉唑处理组予15 mg/kg剂量雷贝拉唑进行灌胃,1次/d,其余组大鼠同时予以等体积生理盐水灌胃,连续给药4 d。以上所有大鼠每次灌胃液体量均按10 mL/kg计算。末次灌胃后所有大鼠禁食不禁水18 h,以10%水合氯醛按3 mL/kg行腹腔注射麻醉处死动物,取小肠组织。
1.2.3 大体观察及损伤评分:沿肠系膜对侧切开小肠,PBS冲洗干净后,于解剖显微镜(10×)下观察,按照修改后的Reuter方法进行小肠损伤情况评分:未见明显损伤为0分;局灶性充血,但未见溃疡形成为1分;有溃疡形成,但无充血及肠管增厚为2分;溃疡形成伴炎性反应(一处)为3分;溃疡形成伴炎症反应(两处及两处以上)为4分;当主要病变部位长度在1~2 cm之间为5分;当主要病变部位长度>2 cm时为6分;当肠管出现轻度粘连(很容易解除的粘连)时计分增加1分,出现明显粘连时计分增加2分。
1.2.4 组织学观察及病理学计分:将小肠组织4%中性甲醛固定24 h,经常规脱水、透明、石蜡包埋切片,HE染色,光镜下观察小肠组织的病理学变化,按照Chiu方法进行病理损伤评分:0分(肠黏膜绒毛正常);1分(绒毛顶端上皮下出现囊状间隙,伴毛细血管充血);2分(上皮下间隙扩大,固有层中度水肿,中央乳糜管扩张);3分(固有层明显水肿,肠黏膜上皮层细胞变性坏死,少数绒毛顶端上皮脱落);4分(上皮层细胞变性坏死、脱落,部分绒毛脱落,固有层裸露,毛细血管扩张、充血);5分(绒毛脱落,固有层崩解,出血或溃疡形成)。
1.2.5 Occludin免疫组织化学染色:采用 Envision法,石蜡切片脱蜡至水,蛋白酶E 37℃ 10 min消化,PBS冲洗,加 Occludin抗体(1∶60)4℃过夜,PBS冲洗,加Envision二抗37℃ 30 min,PBS冲洗,DAB显色,镜下控制显色时间,苏木精复染、脱水、透明、树胶封片。观察染色为棕褐色的阳性细胞的百分比进行综合判定:阳性细胞数<10%为阴性(-),10% ~20%为弱阳性(+),20% ~50%为中等阳性(++),>50%为强阳性(+++)。
1.2.6 Western blot分析:取约 100 mg 小肠组织,加入蛋白裂解液,4℃裂解,10 000 r/min离心15 min,取上清为全蛋白提取物,将蛋白提取物与蛋白上样缓冲液混合,煮沸5 min,分装保存于-80℃冰箱。进行SDS-PAGE凝胶电泳、转膜,封闭后加入一抗4℃过夜。再加入辣根过氧化物酶标记的二抗,孵育后ECL法显色于X光片后照相,以β-actin作为内参照,使用Image J 1.44p软件分析目的蛋白条带灰度值与内参照β-actin条带灰度值的比值,作为目的蛋白相对表达量。
1.3 统计学处理 采用SPSS v17.0软件进行处理及分析,各组损伤指数以中位数表示,统计数据采用两个独立样本的秩和检验,统计量采用Mann-Whitney U检验;Western blot实验数据用x-±s表示,统计数据采用两个独立样本的t检验;P<0.05为差异有统计学意义。
2 结果
2.1 各组大鼠小肠组织大体及病理学观察
2.1.1 大体观察:阴性对照组大鼠小肠组织未见明显损伤;双氯酚酸损伤组小肠损伤较重,腹腔内可见肠管粘连、水肿、扩张,小肠黏膜可见多处散发充血、糜烂及片状溃疡;而雷贝拉唑处理组肠管粘连及水肿不明显,小肠黏膜可见少量散在点状糜烂及小溃疡。
2.1.2 病理观察:阴性对照组黏膜上皮结构完整,形态正常,未见溃疡及糜烂;双氯酚酸损伤组小肠绒毛结构消失,可见坏死、糜烂及溃疡形成,固有层可见炎细胞浸润,部分黏膜上皮结构完全破坏;而雷贝拉唑处理组也可见部分坏死及溃疡形成,但较损伤组明显减轻,大部分黏膜上皮结构尚完整。
2.1.3 大体及病理学损伤评分:双氯酚酸损伤组大体和病理评分明显高于阴性对照组(P=0.000);雷贝拉唑处理组大体和病理评分与损伤组相比均降低,差异具有统计学意义(P<0.05,见表1)。
2.2 小肠组织Occludin蛋白免疫组织化学染色 根据免疫组织化学染色结果,阴性对照组中Occludin蛋白分布于小肠黏膜上皮细胞膜,呈表达强棕褐色信号,强阳性表达率为60%(6/10);损伤组Occludin蛋白表达明显减弱,呈低表达,强阳性表达率为10%(1/10);雷贝拉唑处理组可观察到Occludin蛋白的棕褐色信号表达较损伤组高,强阳性表达率为40%(4/10)(见图1、表2)。
2.3 Western blot检测小肠组织Occludin和p-ERK蛋白表达水平 损伤组小肠组织Occludin蛋白的表达明显低于对照组(P<0.05),而雷贝拉唑处理组Occludin蛋白的表达高于损伤组(P<0.05);损伤组中p-ERK蛋白的表达明显高于对照组(P<0.05),而雷贝拉唑处理组p-ERK蛋白的表达低于损伤组(P<0.05,见图 2、表 3)。
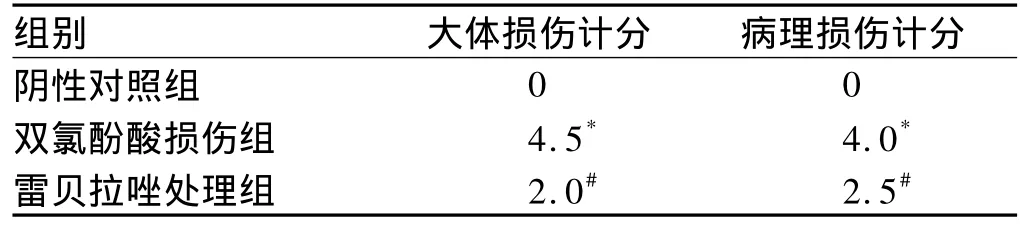
表1 各组大鼠小肠组织大体及病理损伤计分中位数比较(n=10)Tab 1 Comparison of general and pathological injury scores in each group(n=10)

图1 各组小肠组织Occludin蛋白免疫组织化学染色表达水平(100×)A:阴性对照组;B:双氯酚酸损伤组;C:雷贝拉唑处理组Fig 1 Expression of Occludin protein immunohistochemical staining in each group(100×) A:negative control group;B:cliclofenal injury group;C:Kabeprazole treated group

表2 各组免疫组织化学胃黏膜组织Occludin蛋白表达水平Tab 2 Comparison of expression of Occludin protein immunohistochemical staining in each group
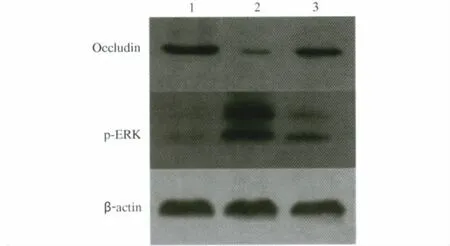
图2 各组小肠组织Occludin和p-ERK蛋白Western blot表达结果 1:阴性对照组;2:双氯酚酸损伤组;3:雷贝拉唑处理组Fig 2 ExpressionResultsof Occludin and p-ERK protein for small intestinal tissue in each group by Western blot 1:negative control group;2:diclofenal injury group;3:rabeprazole treated group
表3 各组大鼠小肠组织 Occludin/β-actin和p-ERK/β-actin表达水平比较(±s)Tab 3 Comparison of Occludin/β-actin and p-ERK/β-actin expression for rat intestinal tissue in each group(±s)
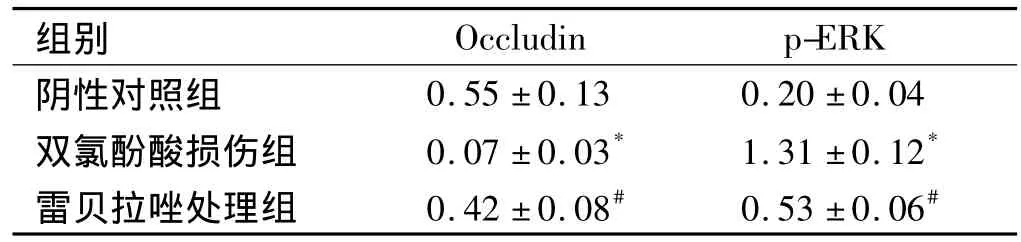
表3 各组大鼠小肠组织 Occludin/β-actin和p-ERK/β-actin表达水平比较(±s)Tab 3 Comparison of Occludin/β-actin and p-ERK/β-actin expression for rat intestinal tissue in each group(±s)
与阴性对照组相比,*P<0.05;与双氯酚酸损伤组相比,#P<0.05
组别Occludin p-ERK阴性对照组0.55 ±0.13 0.20 ±0.04双氯酚酸损伤组 0.07 ±0.03* 1.31 ±0.12*雷贝拉唑处理组 0.42 ±0.08# 0.53 ±0.06#
3 讨论
近来有研究发现,质子泵抑制剂(PPI)不仅能够减少胃酸分泌,而且具有抗炎抗氧化的作用[2]。研究表明,PPI对NSAIDs相关性胃损伤具有保护作用[3-5],而对NSAIDs相关性小肠损伤的影响并未取得统一意见:有部分研究者认为,PPI对NSAIDs相关性小肠损伤具有保护作用,其中以兰索拉唑、雷贝拉唑效果较明显[6-8];另有部分研究者却发现,PPI与NSAIDs合用时对小肠的损伤较单用NSAIDs时更严重[9-10]。临床中NSAIDs经常需要与PPI联用,因此明确PPI对NSAIDs相关性小肠损伤的影响对指导临床用药具有很大的意义。
本实验参考了陈汉卿等[8]以双氯酚酸制造大鼠NSAIDs相关性小肠损伤模型的方法,减少双氯酚酸的用药时间(由5 d减少为4 d),实验过程中未发现有大鼠提前死亡的情况,处死后亦可观察到大鼠明显的小肠损伤,造模成功。实验结果发现,与损伤组相比,雷贝拉唑处理组大鼠小肠的大体和病理损伤情况均明显减轻(P<0.05)。因此,在本实验,我们认为雷贝拉唑对NSAIDs相关性小肠损伤有保护作用。
Occludin蛋白是紧密连接中最重要的组成蛋白之一,对紧密连接的构成、黏膜屏障的维持起关键作用。在肠黏膜上皮中,当Occludin蛋白表达发生变异、减少、缺失时,可导致肠黏膜屏障功能障碍,黏膜通透性增高,从而引起小肠黏膜损伤[11-12]。本实验中,在双氯酚酸损伤后,大鼠小肠组织中Occludin蛋白表达明显下降,而经过雷贝拉唑处理的大鼠,Occludin蛋白则较损伤组上调表达,与大体及病理结果一致,提示雷贝拉唑的保护作用与上调小肠黏膜中Occludin蛋白表达有关。
细胞外信号调节激酶/丝裂原活化蛋白激酶通路(ERK/MAPK)是经典的细胞信号转导途径,具有调节细胞增殖、分化、凋亡等多重功能。ERK/MAPK信号通路在NSAIDs相关性消化道损伤过程中扮演重要角色,ERK/MAPK信号通路的激活参与了NSAIDs相关性胃肠道损伤的过程[13-14]。另有研究[15-16]发现,ERK信号通路对包括Occludin在内的多种紧密连接蛋白的表达起到重要的调节作用,ERK蛋白能够通过自身磷酸化的过程调控Occludin蛋白的表达,进而影响黏膜屏障功能。结合本实验中ERK蛋白在损伤组磷酸化程度明显增加,而处理组的磷酸化程度较损伤组减轻,我们认为,ERK/MAPK信号通路参与了雷贝拉唑对大鼠小肠黏膜的保护过程,可能与调节紧密连接蛋白Occludin的表达有关。
基于以上结果,我们认为,雷贝拉唑对大鼠NSAIDs相关性小肠损伤具有保护作用,其可能是通过MAPK中ERK途径,上调紧密连接蛋白Occludin的表达,进而增强肠上皮黏膜屏障功能而实现的。本实验仅对雷贝拉唑的肠黏膜保护作用进行了初步探索,其他质子泵抑制剂是否具有同样的保护作用和机制,仍需后续研究进一步证实。
[1] Adebayo D, Bjarnason I. Is non-steroidal anti-inflammaory drug(NSAID)enteropathy clinically more important than NSAID gastropathy?[J].Postgrad Med J,2006,82(965):186-191
[2] Kedika RR,Souza RF,Spechler SJ.Potential anti-inflammatory effects of proton pump inhibitors:a review and discussion of the clinical implications[J].Dig Dis Sci,2009,54(11):2312-2317.
[3] Genta RM,Rindi G,Fiocca R,et al.Effects of 6-12 months of esomeprazole treatment on the gastric mucosa [J].Am J Gastroenterol,2003,98(6):1257-1265.
[4] Stupnicki T,Dietrich K,González-Carro P,et al.Efficacy and tolerability of pantoprazole compared with misoprostol for the prevention of NSAID-related gastrointestinal lesions and symptoms in rheumatic patients[J].Digestion,2003,68(4):198-208.
[5] Laheij RJ,Van Rossum LG,Jansen JB,et al.Proton-pump inhibitor therapy for acetylsalicylic acid associated upper gastrointestinal symptoms:a randomized placebo-controlled trial[J].Aliment Pharmacol T-her,2003,18(1):109-115.
[6] Yoda Y,Amagase K,Kato S,et al.Prevention by lansoprazole,a proton pump inhibitor,of indomethacin-induced small intestinal ulceration in rats through induction of heme oxygenase-1[J].J Physiol Pharmaeol,2010,61(3):287-294.
[7] Pozzoli C,Menozzi A,Grandi D,et al.Protective effects of proton pump inhibitors against indomethacin-induced lesions in the rat small intestine [J].Naunyn Schmiedebergs Arch Pharmacol,2007,374(4):283-291.
[8] Chen HQ,Lv B,Chen MY,et al.The study on the protection of proton pump inhibitors in diclofenac induced small intestinal injury[J].Chin J Dig,2011,31(11):750-765.
陈汉卿,吕宾,陈鸣艳,等.质子泵抑制剂对双氯酚酸诱导小肠黏膜损伤保护机制研究[J].中华消化杂志,2011,31(11):750-756.
[9] Goldstein JL,Eisen GM,Lewis B,et al.Investigators.Video capsule endoscopy to prospectively assess small bowel injury with celecoxib,naproxen plus omeprazole and placebo[J].Clin Gastroenterol Hepatol,2005,3(2):133-141.
[10] Wallace JL,Syer S,Denou E,et al.Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis[J].Gastroenterology,2011,141(4):1314-1322.
[11] Berkes J,Viswanathan VK,Savkovic SD,et al.Intestinal epithelial responses to enteric pathogens:effects on the tight junction barrier,ion transport,and inflammation [J].Gut,2003,52(3):439-451.
[12] Mitic LL,Van Itallie CM,Anderson JM.Molecular physiology and pathophysiology of tight junctions I.Tight junction structure and function:lessons from mutant animals and proteins[J].Am J Physiol Gastrointest Liver Physiol,2000,279(2):G250-G254
[13] Pan MR,Chang HC,Hung WC.Non-steroidal anti-inflammatory drugs suppress the ERK signaling pathway via block of Ras/c-Raf interaction and activation of MAP kinase phosphatases[J].Cell Signal,2008,20(6):1134-1141.
[14] Samak G,Aggarwal S,Rao RK.ERK is involved in EGF-mediated protection of tight junctions,but not adherens junctions,in acetaldehyde-treated Caco-2 cell monolayers[J].Am J Physiol Gastrointest Liver Physiol,2011,301(1):G50-G59.
[15] Basuroy S,Seth A,Elias B,et al.MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen Peroxide[J].Bioehem J,2006,393(Pt 1):69-77.
[16] Yang R,Harada T,Li J,et al.Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase l/2 dependent mechanism[J].Intensive Care Med,2005,31(5):709-717.
The effects and mechanism of rabeprazole on tight junction protein occludin expression in rats with non-steroid anti-inflammatory drugs(NSAIDs)induced small intestinal injury
GAO Xin1,ZHANG Zhenyu1,WU Hailu1,HU Kewei1,JIANG Zongdan1,YANG Xiaobing2,WANG Jinsong2
1.Department of Gastroenterology;2.Department of Pathology,Nanjing First Hospital Affiliated to Nanjing Medical University,Nanjing 210006,China
ObjectiveTo explore the effects and mechanism of rabeprazole on tight junction protein occludin expression in rats with non-steroid anti-inflammatory drugs(NSAIDs)induced small intestinal injury.Methods36 SD rats were randomly and equally divided into control group,injury group and rabeprazole treated group.Except control group,rats of other two groups were gavaged with diclofenac 7.5 mg/(kg·d),once daily to make NSAIDs related small intestinal injury model.The treated group was gavaged with rabeprazola 15 mg/(kg·d)once daily 0.5 h before the administration of diclofenac.Continuous administration for four days and then executed,small intestinal tissues were taken and observed for gross and pathology changes.Immunohistochemistry and Western blot were used to detect the distribution and expression of intestinal epithelial tight junction protein occludin.The expression of phosphorylation-ERK(p-ERK)was determined by Western blot.ResultsCompared with injury group,the gross and tissue injury scores of rabeprazole treated group significantly decreased(P <0.05).The expression of occludin in injury group was decreased significantly compared with normal group(P <0.05),however,rabeprazole treated group decreased lightly(P <0.05).Significant activation of ERK were more obvious in injury group than that in normal group(P <0.05),but pretreatment with rabeprazole could inhibit the activation of ERK(P <0.05).ConclusionRabeprazole has a protective effect on NSAIDs induced small intestinal injury in rats,probably increasing the expression of tight junction protein occludin by acting ERK signaling pathways.
Rabeprazole;NSAIDs related small intestinal injury;Occludin;MAPK/ERK
R574
A
1006-5709(2012)08-0771-04
2012-01-27
10.3969/j.issn.1006-5709.2012.08.025
高欣,在读硕士。E-mail:gx_198824@sina.com
张振玉,E-mail:ahwangzhibing776@163.com

