Liver transplantation in Crigler-Najjar syndrome type I disease
Hangzhou,China
Introduction
Crigler-Najjar syndrome type I (CNS I) disease,a very rare autosomal recessive inherited disease characterized by severe indirect hyperbilirubinemia from birth with normal liver function,is due to lack of activity of hepatic enzyme uridine diphosphate (UDP)-glucuronosyltransferase.Phototherapy is effective to reduce the bilirubin level of infants in the early phase of treatment,but later the patients are still at high risk of kernicterus and neurological sequels that can be irreversed even after liver transplantation (LTx).This consequence can ultimately lead to severe disability or death.[1,2]LTx,a life-saving procedure,may be a promising alternative technique to treat liver-based inborn errors of metabolism,for instance,CNS I.
Case report
A full-term male infant was detected with a high concentration of indirect bilirubin on his third day after birth.The infant was born in a family with a history of hyperbilirubinemia.A similar disorder brought death to his elder sister at age of six.His parents carry raised serum bilirubin levels,about 35-45 μmol/L,composed of the indirect bilirubin level about 27-38 μmol/L.His elder brother grows healthily.After thefinal diagnosis,the infant was treated with phototherapy 4-8 hours every other day during 7 months as a course.Consequently this treatment was less effective and canceled.Phenobarbital was taken orally 15 mg/d in an attempt to prevent convulsion during the 4th to 8th postnatal weeks.On admission to our center,he was 18 months old,85 cm in height,and 11.5 kg in weight.He didn't suffer from any fever or infectious disease until the appearance of sudden symptoms of kernicterus,including bylethargy,hypertonia,mild neck rigidity,and subsequent athetosis.Other symptoms,such as high-pitched cry and arching of the back (retrocollis or opisthotonus) were negative.The total bilirubin level rose progressively from 552.4 to 742.0 μmol/L before transplantation.The infant underwent a left lateral lobe LTx with a viable and high-quality liver obtained from a volunteer.
Genetic detecting
Genomic DNA was extracted from peripheral blood using QIAamp DNA Blood Mini Kit (QIAGEN).After purification,PCR amplification was performed.The primer sequences were:5'-GCC AGT TCA ACT GTT GTT GCC-3' and 5'-CCA CTG GGA TCA ACA GTA TCT-3'.The PCR conditions were as follows:94 ℃ for 2 minutes,30 cycles of 94 ℃ for 15 seconds,58 ℃ for 30 seconds,and 68 ℃ for 30 seconds,and afinal elongation at 68 ℃ for 5 minutes.DNA sequencing was performed using a 377 automatic sequencer (Applied Biosystems).The TATAA element is the binding site for transcription factor IID,which is important during the transcriptional initiation of the gene UDP-glucuronosyltransferase.[3]As shown in Fig.1,the genotype of proband was homozygous for A(TA)7/7TAA,and the genotypes of his parents were all heterozygous for A(TA)6/7TAA.The mutation of TATAA element can reduce the frequency and accuracy of transcription initiation.The presence of this longer A(TA)7/7TAA element in the promoter region of the gene for bilirubin UDP-glucuronosyltransferase resulted in reduced expression of a reporter gene in a human hepatoma cell line.[3]
Graft
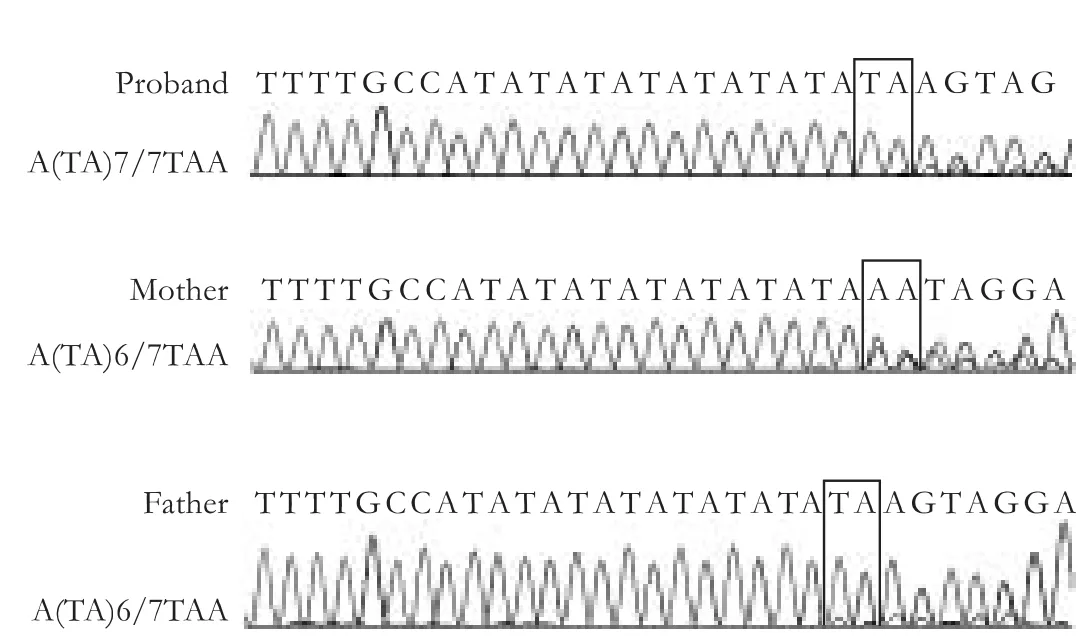
Fig.1.The results of TATA-box genotype in the promoter region.The genotype of proband was homozygous for A(TA)7/7TAA,the genotypes of his parents were all heterozygous for A(TA)6/7TAA.
The graft was donated by a non-heart-beating voluntary donor following the guidelines of the Ethics Committee of our hospital and the Declaration of Helsinki.The donor's liver was divided into two parts:the left part (segments II and III) was suitably transplanted into the child,while the right part into another adult recipient simultaneously.The graft weighed 262 g,accounting for 72% of estimated standard liver mass (364 g),according to the formula proposed to estimate adult and pediatric normal liver size.[4]
Motor development
Motor development was assessed by the Peabody Development Motor Scales (PDMS-2),including gross motor quotient (GMQ),fine motor quotient (FMQ)and total motor quotient (TMQ).The normative sample consisted of 2003 children,who were developing typically from 0 to 71 months of age,and from the United States and one of the Canadian provinces.Results from PDMS-2 revealed that the recipient obtained slightly higher scores after LTx in GMQ and FMQ respectively.However,the recipient's PDMS-2 results still remained delayed motor developments,compared with those of average children.
Magnetic resonance imaging (MRI) and magnetic resonance spectroscopy (MRS)

Fig.2.Axial T2-weighted MR images show bilateral frontal horns became blunt.A:pre-operation; B:1-month post-operation; C:3-month post-operation; D:1-year post-operation.
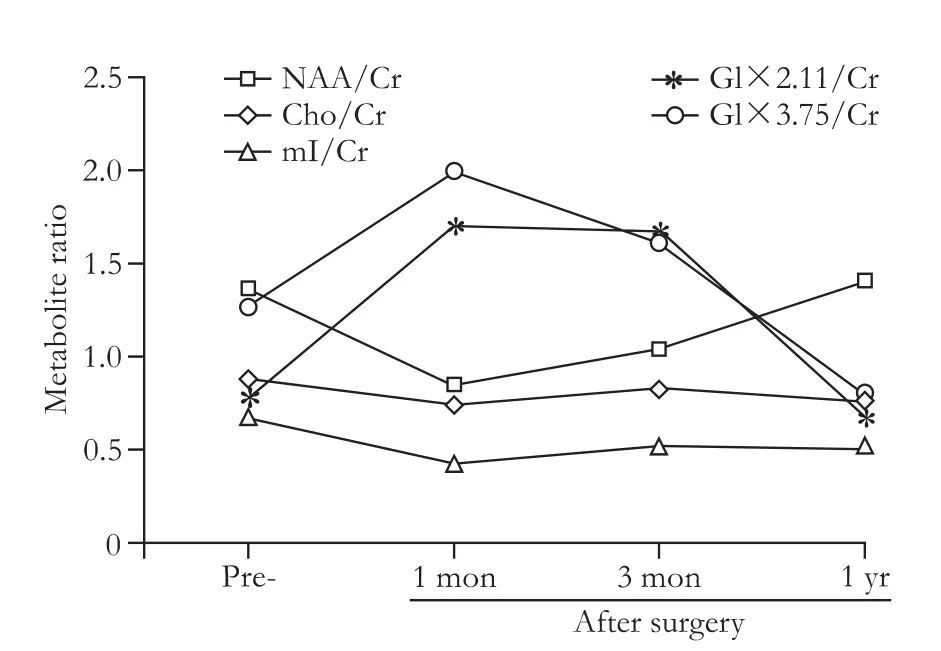
Fig.3.Metabolite ratios of MRS from the right basal ganglia.NAA:N-acetyl-aspartate; Cr:creatine; Cho:choline; mI:myo-Inositol;Glx:glutamine.
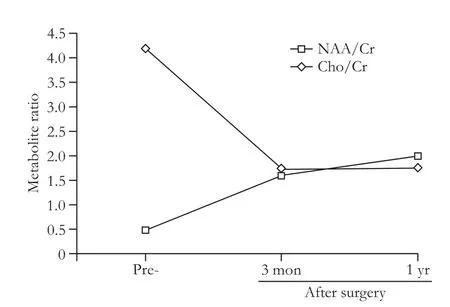
Fig.4.Metabolite ratios of MRS from the right periventrical white matter.NAA:N-acetyl-aspartate; Cr:creatine; Cho:choline.
MRI was performed before LTx and 1 month,3 months,and 1 year after surgery.Symmetrical white matter hyperintensity was obtained around the ventricles.Symmetrical periventricular hyperintensity was also shown on the postoperative follow-up images.Recovery of abnormal signal was very slow until one year later,and only MRI-T2-weighted visible reduction was seen in some abnormal signal (Fig.2).Conventional MRI showed significant improvement in a one-year followup.MRS followed by MRI was also studied.Short TE(TE=8-10 msec) STEAM sequences were localized in the bilateral basal ganglia (BG) and the cingulate gyrus respectively.Long TE (TE=144 msec) PRESS sequences were localized at the periventricular white matter(PvWM) hyperintensity region.Only the right BG and right PvWM of the spectral data were completed.MRS results of the right BG showed that NAA/Cr was increased in one-way,and mI/Cr,Glx/Cr,and Cho/Cr ratios had a downward trend (Fig.3).Right PvWM metabolites showed an increasing trend of NAA/Cr and a decreasing trend of Cho/Cr (Fig.4).The results showed that brain pathology had a trend to return to normal.

Fig.5.Changes of total bilirubin level and indirect bilirubin level.Day 0 donates the operation day.
Outcome
The total bilirubin level fell to 292.5 μmol/L 8 hours after operation.The serum bilirubin level was progressively decreasing,though there was considerable mild fluctuation during the firstfive days without any inducement (Fig.5).Mental manifestation was recovered after transplantation and no signs of kernicterus were detected.To date,no recurrence of other symptoms has been diagnosed.
Discussion
One multi-center study showed kernicterus would develop in a number of patients while serum bilirubin level is at least above 500 μmol/L.[5]In this case,the patient developed kernicterus suddenly with the raised total bilirubin level as high as 700 μmol/L.Phototherapy is considered effective in controlling neonatal indirect hyperbilirubinaemia.[1]However,it is less effective because of the body surface/weight ratio,which requires increased time for the therapy.
When signs of acute kernicterus appear in a jaundiced infant,permanent brain damage takes place.Immediate treatment should be prescribed to prevent further damage because certain types of damage are possibly reversible.Currently,LTx is the most efficient method to improve the quality of life of patients for a medium period of time.However,timing of LTx is difficult because the onset of bilirubin encephalopathy is unpredictable,and the best age for transplantation remains under discussion.Our patient received LTxfive days after kernicterus.It is recognized that patients with CNS I should undergo LTx at a young age to reduce the high risk of brain damage.
MRI of infants with kernicterus usually shows abnormal changes in signal intensity in various parts of the brain,including the globus pallidus and subthalamic regions.[6,7]In our patient,preoperative MRI showed symmetric white matter hyperintensities around the periventricular region,which could be an untypical type of imagings.Kernicterus has a characteristic signature on 1H-MR spectroscopy.[8]The results of this study agree with those about bilirubin encephalopathy.The one-year imaging follow-up showed better prognosis by declined periventricular abnormal hyperintensity and increased NAA activity.MRI revealed positive changes one year after operation while MRS showed an improved trend in a short period.MRS displayed findings earlier than MRI in monitoring patients with bilirubin encephalopathy.MRS could be an option for predicting brain conditions and determining patients with CNS I for transplantation.A large number of patients are required to determine the correlation between brain metabolite levels and clinical outcomes.
Most of the recipients are considered to have normal neuro-development.About 36% of CNS I patients with brain damage show marked improvement in neurological symptoms after LTx.[9]Our patient suffered from brain damage defined by symptoms and neurological findings.Motor development estimated by PDMS-2 showed higher scores after transplantation,suggesting that neurological changes are likely to be reversed.Early neurological symptoms of bilirubin toxicity can be improved if the treatment is intensified immediately.Full-blown kernicterus is irreversible,but symptoms of sudden kernicterus are reversible.[9]In our case,the neurological status of the recipient was successfully reversed after LTx.However,long-term observation is needed to obtain long-term outcome.
Auxiliary partial orthotopic LTx and liver cell transplantation are introduced as curative approaches.[10-12]However,the procedures have not been widely used because of their limited indications or therapeutic effects.
In summary,LTx can produce acceptable survival outcomes and excellent quality of life for patients with CNS I.Combined therapeutic strategies would be the direction of treatment in the future.In our case,symptoms of the recipient were successfully reversed after healing of brain damage.Besides,MRS as a more sensitive technique than MRI is indicated for monitoring brain conditions,and a prospective multi-center study of the timing of transplantation is demanded.
Acknowledgements:We thank Sheng Yan,Xue-Juan Zhou,Lu Zhang,Lun-Jie Luo,Lin Zhang and Ji-Lei Fu for technical assistance.
Contributors:ZSS proposed the study.TZH,SDS and ZW performed research and wrote the first draft.JJC and LHY collected and analyzed the data.WWL and ZM performed the operation.All authors contributed to the design and interpretation of the study and to further drafts.ZSS is the guarantor.
Funding:This study was supported by grants from Team Program of Science and Technology Bureau of Zhejiang Province (2009R50038)and National Key Technology R&D Program (2008BAI60B02).
Ethical approval:The graft was donated by a non-heart-beating voluntary donor following the guidelines of the Ethics Committee of our hospital and the Declaration of Helsinki.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Yohannan MD,Terry HJ,Littlewood JM.Long term phototherapy in Crigler-Najjar syndrome.Arch Dis Child 1983;58:460-462.
2 Ozçay F,Alehan F,Sevmiş S,Karakayali H,Moray G,Torgay A,et al.Living related liver transplantation in Crigler-Najjar syndrome type 1.Transplant Proc 2009;41:2875-2877.
3 Bosma PJ,Chowdhury JR,Bakker C,Gantla S,de Boer A,Oostra BA,et al.The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome.N Engl J Med 1995;333:1171-1175.
4 Urata K,Kawasaki S,Matsunami H,Hashikura Y,Ikegami T,Ishizone S,et al.Calculation of child and adult standard liver volume for liver transplantation.Hepatology 1995;21:1317-1321.
5 van der Veere CN,Sinaasappel M,McDonagh AF,Rosenthal P,Labrune P,Odievre M,et al.Current therapy for Crigler-Najjar syndrome type 1:report of a world registry.Hepatology 1996;24:311-315.
6 Sugama S,Soeda A,Eto Y.Magnetic resonance imaging in three children with kernicterus.Pediatr Neurol 2001;25:328-331.
7 Katar S,Akay HO,Taskesen M,Devecioglu C.Clinical and cranial magnetic resonance imaging (MRI) findings of 21 patients with serious hyperbilirubinemia.J Child Neurol 2008;23:415-417.
8 Oakden WK,Moore AM,Blaser S,Noseworthy MD.1H MR spectroscopic characteristics of kernicterus:a possible metabolic signature.AJNR Am J Neuroradiol 2005;26:1571-1574.
9 Schauer R,Stangl M,Lang T,Zimmermann A,Chouker A,Gerbes AL,et al.Treatment of Crigler-Najjar type 1 disease:relevance of early liver transplantation.J Pediatr Surg 2003;38:1227-1231.
10 Rela M,Muiesan P,Vilca-Melendez H,Dhawan A,Baker A,Mieli-Vergani G,et al.Auxiliary partial orthotopic liver transplantation for Crigler-Najjar syndrome type I.Ann Surg 1999;229:565-569.
11 Lysy PA,Najimi M,Stephenne X,Bourgois A,Smets F,Sokal EM.Liver cell transplantation for Crigler-Najjar syndrome type I:update and perspectives.World J Gastroenterol 2008;14:3464-3470.
12 Meyburg J,Schmidt J,Hoffmann GF.Liver cell transplantation in children.Clin Transplant 2009;23:75-82.
 Hepatobiliary & Pancreatic Diseases International2012年5期
Hepatobiliary & Pancreatic Diseases International2012年5期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Disease spectrum and use of cholecystolithotomy in gallstone ileus
- Xanthogranulomatous cholecystitis mimicking gallbladder cancer and causing obstructive cholestasis
- High-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients awaiting liver transplantation
- Laparoscopic distal pancreatectomy with or without splenectomy:spleen-preservation does not increase morbidity
- Expression of HBx protein in hepatitis B virusinfected intrahepatic cholangiocarcinoma
- Effect of endogenous hypergastrinemia on gallbladder volume and ejection fraction in patients with autoimmune gastritis
