卵巢恶性肿瘤组织叶酸结合蛋白表达检测及其临床意义
黄明钜 王琪 张玮 尹巧云 李状 李力
广西医科大学附属肿瘤医院妇瘤科,基础实验部,广西 南宁 530021
卵巢恶性肿瘤组织叶酸结合蛋白表达检测及其临床意义
黄明钜 王琪 张玮 尹巧云 李状 李力
广西医科大学附属肿瘤医院妇瘤科,基础实验部,广西 南宁 530021
背景与目的:叶酸结合蛋白(folate binding protein,FOLR1)又称为叶酸受体蛋白,对细胞分裂、增殖和生长有着非常重要的作用。FOLR1在正常组织中表达较低,但在卵巢癌中存在过度表达。本研究探讨卵巢恶性肿瘤组织中FOLR1的表达及其临床意义。方法:采用Western blot检测80例卵巢恶性肿瘤组织、50例卵巢良性肿瘤组织及30例正常卵巢组织中的FOLR1表达情况,分析其与卵巢恶性肿瘤临床病理及多药耐药的相关性。结果:FOLR1在正常卵巢组织、卵巢良性肿瘤组织和卵巢恶性肿瘤组织中的表达量依次增高(P<0.01)。FOLR1在卵巢恶性肿瘤临床分期中Ⅰ~Ⅱ期的表达量低于Ⅲ~Ⅳ期(P<0.05)。FOLR1在铂类药物耐药型卵巢恶性肿瘤中的表达量低于铂类药物敏感型(P<0.01);治疗后肿瘤进展者的表达量低于缓解者(P<0.01)。FOLR1在黏液性肿瘤中的表达量低于浆液性肿瘤(P<0.05),恶性卵巢肿瘤有淋巴结和远处器官转移的高于无转移者(P<0.05),其表达量与患者的不同肿瘤病理分类、是否有大网膜转移和腹水量无明显相关(P>0.05)。取FOLR1表达量界值为3.115时来判断卵巢肿瘤性质,ROC曲线的Youden指数最大,卵巢恶性肿瘤FOLR1表达量的高低与中位生存时间长短差异无明显相关(P>0.05),Cox模型多因素分析显示,FOLR1表达量不是影响患者生存预后的独立因素。结论:FOLR1可作为一种新型的卵巢恶性肿瘤早期诊断标志物,并可成为判断卵巢恶性肿瘤多药耐药潜在标志之一。
卵巢恶性肿瘤; 叶酸结合蛋白; 耐药
叶酸结合蛋白(folate binding protein,FOLR1)又称为叶酸受体蛋白,是一种糖基磷酸肌醇(glycosylphosphatidylinsitol,GPI)锚定膜蛋白,可介导细胞外叶酸进入细胞内,对细胞分裂、增殖和组织生长有非常重要的作用[1]。FOLR1在正常组织表达较低,但在许多肿瘤细胞如卵巢癌、乳腺癌、肾癌、结肠癌、肺癌、睾丸癌、室鼓膜瘤和脉络膜瘤等存在过度表达[2-4],特别是在卵巢恶性肿瘤中约90%的上皮癌呈高表达[5]。本研究旨在探讨FOLR1表达量与卵巢恶性肿瘤预后、铂类多药耐药等临床指标的相关性。
1 资料和方法
1.1 临床资料
1.1.1 研究对象
样本来源于广西医科大学附属肿瘤医院妇瘤科行手术治疗的患者,均经病理学检查确诊。卵巢恶性肿瘤80例,年龄13~76岁,平均41.1岁。采用世界卫生组织(WHO,1973)制定的卵巢组织学分类法,其中黏液性癌24例,浆液性癌35例,内膜样癌2例,生殖细胞肿瘤11例,性索间质肿瘤3例及其他转移肿瘤5例;临床分期按FIGO(2004)标准,Ⅰ~Ⅱ期30例,Ⅲ~Ⅳ期50例。所有患者均接受肿瘤减灭手术,61例上皮性肿瘤和19例非上皮性肿瘤术后分别接受顺铂+紫杉醇和顺铂+博来霉素+长春新碱的联合化疗。卵巢良性肿瘤50例,年龄10~74岁,平均40.1岁。其中黏液性瘤11例,浆液性瘤36例,畸胎瘤3例。正常卵巢组织30例,年龄29~60岁,平均43.1岁。其中子宫肌瘤28例,宫颈癌1例,乳腺癌1例同时行卵巢切除(征得患者同意),病理证实无异常者。所有的组织标本均在手术时收集,取肿瘤的原发病灶组织,转移灶组织分别置于液氮罐内保存,准备用于提取蛋白和4%的甲醛溶液固定切片行组织病理学检查,研究经本院伦理学委员会批准并征得患者同意。
1.1.2 方法仪器与试剂
组织蛋白裂解液购自上海基星生物有限公司,BCA蛋白浓度测定试剂盒购自Thermo公司,FOLR1和GAPDH羊抗人一抗购自Santa Cruz Biotechnology公司,PVDF膜购自北京索莱宝科技有限公司,红外荧光染料标记的驴抗羊二抗购自美国LI-COR公司,成像系统为美国LICOR公司的Odyssey双色红外荧光成像系统,图像分析采用LI-COR公司开发的Odyssey软件。
1.2 方法
1.2.1 Western blot检测FOLR1表达
从液氮中取出冻存的组织标本,冰上解冻,用预冷的PBS洗涤2次,充分研磨,加入适量含有PMSF的RAPI裂解液,冰上裂解30 min,于4 ℃、12 000 r/min (r=14.2 cm)离心15 min,取上清液分装,用BAC法测定蛋白浓度。体积按4∶1加1×上样缓冲液混匀,根据测算的浓度确定50 μg为上样量,换算出各自的实际上样体积。用普通PCR仪煮沸5 min变性,进行SDSPAGE电泳分离蛋白(5%浓缩胶,12%分离胶),浓缩胶跑40 V的恒压,分离胶跑80 V的恒压,按照蛋白质Marker切下FOLR1和GAPDH胶段,转移至PVDF膜上(恒100 mA,42 min),5%脱脂牛奶封闭非特异抗原2.5 h,PBS-T洗涤1次,5 min,分别加入1∶800稀释的羊抗人FOLR1多克隆抗体和1∶1 000稀释的羊抗人GAPDH多克隆抗体,室温1 h,4 ℃过夜。PBS-T洗涤4次,每次5 min。再分别加入1∶10 000稀释的红外荧光染料标记的驴抗羊二抗,室温震摇2 h,PBS-T洗涤3次,每次5 min,PBS洗涤1次,10 min,进行双色红外荧光成像扫描和图像条带灰度值的分析。每个样本条带与相对应样本GAPDH条带的灰度值相比,求出其比值即为FOLR1的相对表达量。
1.2.2 生存随访及疗效判断
治疗后通过电话对卵巢恶性肿瘤患者本人或家属进行随访,随访至2010年12月,失访5例,无非癌死亡者。中位随访时间为29.8个月(1~60个月)。以WHO的实体肿瘤疗效标准来评价近期疗效。肿瘤患者铂类耐药或非耐药评判参照文献[6]。
1.3 统计学处理
采用SPSS 17.0软件进行处理,对样本FOLR1与GAPDH相对表达量定量资料以表示,其相对表达量数据进行正态和方差齐性检验,资料呈偏态分布及方差不齐,样本间的FOLR1表达量比较采用完全随机设计多个样本比较的秩和检验,两组样本间的FOLR1表达量比较采用Wilcoxon秩和检验。生存率估计采用Kaplan-Meier法,绘制生存曲线,生存曲线比较采用log-rank检验。生存资料的多因素分析采用Cox模型。FOLR1表达量的灵敏度和特异度以Youden指数为评价指标,采用ROC曲线进行描述。P<0.05为差异有统计学意义。
2 结 果
2.1 FOLR1在正常卵巢、卵巢良性肿瘤和卵巢恶性肿瘤组织中的表达
FOLR1在正常卵巢组织、卵巢良性肿瘤组织和卵巢恶性肿瘤组织中的表达量依次增高,差异均有统计学意义(P<0.01,图1,表1)。
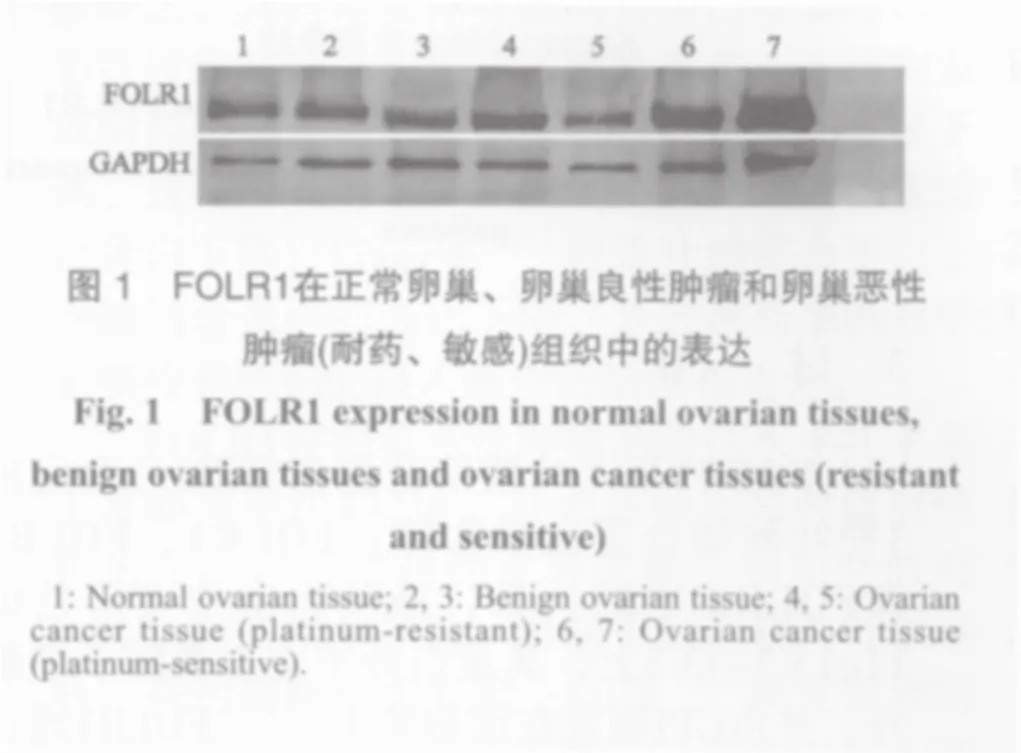
2.2 卵巢恶性肿瘤组织中的FOLR1表达量与临床病理因素的关系
FOLR1在卵巢恶性肿瘤临床分期中Ⅰ~Ⅱ期的表达量低于Ⅲ~Ⅳ期,FOLR1在卵巢恶性肿瘤4种不同病理类型中的表达量差异无统计学意义(P>0.05),但在黏液性肿瘤中的表达量低于浆液性肿瘤,差异有统计学意义(P<0.05,表2)。
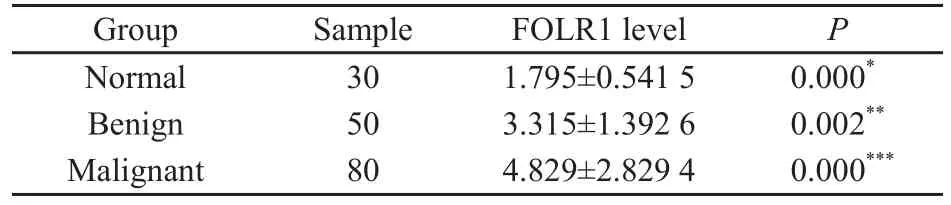
表 1 各类卵巢组织中的FOLR1表达量Tab. 1 FOLR1 levels (gray values) measured in all samples
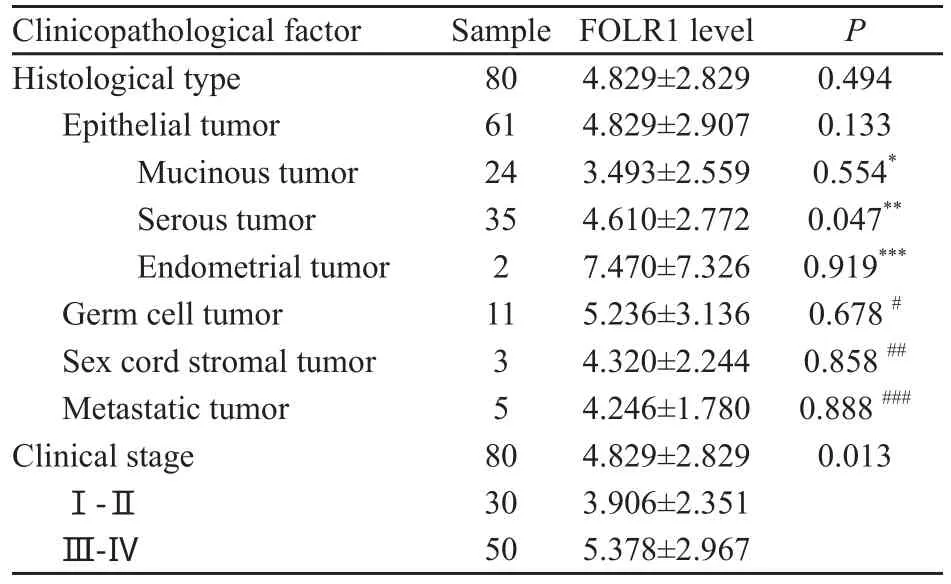
表 2 卵巢恶性肿瘤组织中FOLR1表达量与临床因素的关系Tab. 2 Relationship of FOLR1 expression with clinic alpathological factors in malignant ovarian tissues
2.3 卵巢恶性肿瘤组织中的FOLR1表达量与肿瘤转移、腹水量的关系
FOLR1表达量在有淋巴结和远处器官转移的卵巢恶性肿瘤中高于无转移者,差异有统计学意义(P<0.05),与患者是否有大网膜转移和腹水量无明显相关,差异无统计学意义(P>0.05,表3)。
2. 4 卵巢恶性肿瘤组织中的FOLR1表达量与疗效和耐药的相关性
在疗效达完全缓解(complete response,CR)、部分缓解(partial response,PR)、疾病稳定(stable disease,SD)和疾病进展(progressive disease,PD)患者中,FOLR1的表达量依次降低,差异有统计学意义(P<0.01)。铂类药物耐药型卵巢恶性肿瘤的FOLR1表达量低于铂类药物敏感型,差异有统计学意义(P<0.01,表 4)。
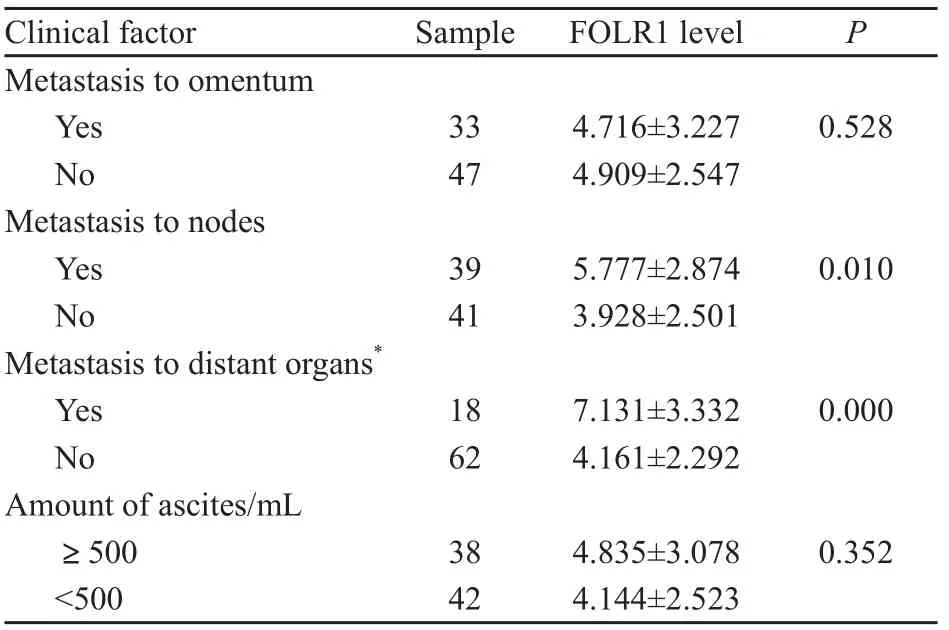
表 3 卵巢恶性肿瘤组织中FOLR1表达量与肿瘤转移、患者腹水量的关系Tab. 3 Relationship of FOLR1 levels with metastasis to lymph nodes and the amount of ascites in ovarian cancer tissues
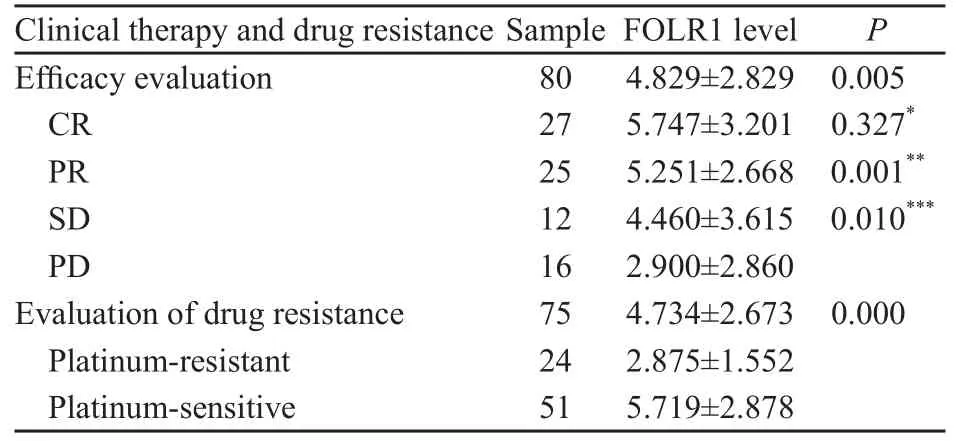
表 4 卵巢恶性肿瘤组织中FOLR1表达量与疗效和耐药的相关性Tab. 4 Association of FORL-1 levels with clinical therapy and drug resistance in ovarian cancers
2.5 卵巢恶性肿瘤组织中的FOLR1表达量与预后的关系
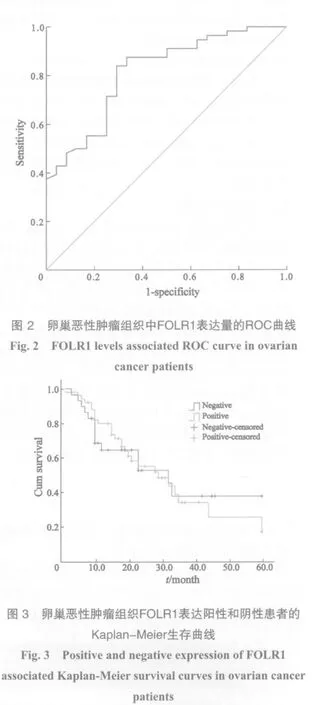
FOLR1表达量用于判断卵巢肿瘤性质的灵敏度和特异度以Youden指数为评价指标,其最大数值所对应的FOLR1表达量为3.115,即表达量>3.115为阳性,<3.115为阴性(图2)。
以FOLR1表达量单因素分析,Kaplan-Meier法绘制生存曲线,log-rank检验FOLR1表达量为阳性患者的中位生存时间为29.4个月,阴性患者为32.3个月。差异无统计学意义(P>0.05,图3)。
采用Cox比例风险回归模型分析显示,FOLR1表达量不是卵巢恶性肿瘤患者生存独立影响因素(表5)。
3 讨 论
FOLR1是人叶酸结合蛋白家族(已鉴定出3种叶酸结合蛋白异构体:FOLR1、FOLR2和FOLR3)中的一员,该基因定位于染色体11q13.3-13.5上,其蛋白分子完全暴露于细胞外,并由GPI锚定在细胞膜上[7]。FOLR1通过胞饮转运机制将细胞外四氢叶酸转运进入细胞内,作为辅酶成分参与DNA复制及损伤修复,其表达水平与肿瘤进展及细胞增殖密切相关[8-9]。本研究发现,卵巢恶性肿瘤中FOLR1高表达,与Dainty等[5]的研究结果一致。Yuan[10]采用定量PCR和流式细胞计数分析发现,在卵巢癌中FOLR1的表达量高于乳腺癌和恶性间皮瘤可成为诊断卵巢恶性肿瘤的标志物。除了传统的方法能鉴定检测FOLR1外,电气化学技术方法也可简单、快速、方便的检测出FOLR1阳性的肿瘤细胞且特异性高[11]。本研究结果还显示卵巢恶性肿瘤Ⅲ~Ⅳ期FOLR1的表达量高于Ⅰ~Ⅱ期,与Toffoli等[12]报道的结论一致。Kimberly等[13]采用免疫组织化学方法对186例原发和27例复发卵巢恶性肿瘤组织,进行单因素和多因素分析发现,FOLR1表达量高低与患者的总体生存时间无关,本研究结果与其相符。据最新报道,中晚期的黏液性卵巢癌对铂类化疗药物的敏感性明显低于浆液性卵巢癌,易发生耐药[14]。已知内源性叶酸缺乏,其转运受体叶酸结合蛋白增高与卵巢恶性肿瘤对铂类药物的敏感性有关,本研究发现耐药型卵巢恶性肿瘤FOLR1的表达量明显下调。这与Shen等[15]发现顺铂耐药细胞叶酸结合蛋白下降;Liang等[16]报道叶酸结合蛋白、砷酸结合蛋白的表达下降,导致耐药细胞对甲氨喋呤和顺铂的摄入减少的结果相吻合。

表 5 Cox模型分析各因素影响卵巢恶性肿瘤预后的地位Tab. 5 analysis on different factors affecting prognosis of ovarian cancers with COX model
FOLR1在卵巢恶性肿瘤的表达水平几乎是正常卵巢组织的2倍,其表达程度和肿瘤对经典化疗的多药耐药性也有关联。利用叶酸对FOLR1的高度亲和性,可用于将药物与叶酸偶联,将药物靶向至肿瘤。Lu等[17]研究发现将对肿瘤细胞分布表达高亲和力的 FOLR1作为靶位,可诱导活化T细胞或提高其靶向、激活T细胞的效率。Roy等[18]发现叶酸和抗TCR/CD3抗体的双特异性偶联物,能特异性结合TCR/CD3复合物,靶向作用于FOLR1能诱发对肿瘤组织强烈的免疫反应,从而介导活化型T细胞的FOLR1阳性肿瘤细胞溶解。目前,卵巢恶性肿瘤化疗药物无明确的靶点和耐药的产生是制约临床治疗效果的主要因素,FOLR1有望成为靶向治疗法的新途径。
卵巢恶性肿瘤发病隐匿,发现时多为晚期,死亡率在全部女性恶性肿瘤中高居第4位,并有逐渐上升的趋势。卵巢恶性肿瘤Ⅲ~Ⅳ期的5年生存率低于30%,Ⅰ~Ⅱ期的5年生存率在60%~90%[19-20]。提高患者早期诊断和无症状者的筛查水平尤为重要,CA125是广泛用于卵巢恶性肿瘤临床诊断的标志物,但在早期患者中表达量不高[21]。Basal等[22]使用Western blot和放射标记法对卵巢恶性肿瘤患者血清进行研究提出FOLR1可作为诊断早期卵巢恶性肿瘤的标志物。如果将CA125联合FOLR1作为卵巢恶性肿瘤早期诊断的标志物,可以进一步提高其诊断的特异度。
卵巢恶性肿瘤的治疗依靠细胞减灭术和以铂类为主的联合化疗,但远期疗效并不理想。Yakirevich等[23]研究发现约75%~80%的卵巢上皮癌开始对化疗有反应,其余则表现为原发耐药,最终所有化疗患者至少80%出现耐药。卵巢恶性肿瘤多药耐药常导致化疗的失败,其多药耐药的形成机制十分复杂,经典的多药耐药机制涉及ABC型膜载体蛋白家族,也与细胞存活通路激活、细胞凋亡通路受阻、p53基因突变及DNA损伤修复系统失调有关。本研究结果发现,耐药型卵巢恶性肿瘤中FOLR1的表达量明显下调,目前尚不明确其表达量的改变是耐药发生的原因还是结果,除此之外还有其他未知结果。因此对未知的机理还需要进一步研究以明确其中的关系及FOLR1的改变对卵巢癌耐药的影响,采取针对性治疗措施防止或逆转耐药的产生,改善化疗效果,对提高患者生存率有着重要的意义。
[1]SIERRA E E,GOLDMAN I D. Recent advances in the understanding of the mechanism of membrane transport of folates and antifolates [J]. Semin Oncol, 1999, 26(Suppl 6):11-23.
[2]PARKER N, TURK M J, WESTRICK E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay [J]. Anal Biochem,2005, 338(2): 284-293.
[3]EVANS C O, YOUNG A N, BROWN M R, et al. Novel patterns of gene expression in pituitary adenomas identified by complementary deoxyribonucleic acid microarrays and quantitative reverse transcription-polymerase chain reaction[J]. J Clin Endocrinol Metab, 2001, 86(7): 3097-3107.
[4]MANTOVANI L T, MIOTTI S, MENARD S, et al. Folate binding protein distribution in normal tissues and biological fluids from ovarian carcinoma patients as detected by the monoclonal antibodies MOv18 and MOv19 [J]. Eur J Cancer, 1994, 30A(3): 363-369.
[5]DAINTY L A, RISINGER J I, MORRISON C, et al.Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma [J]. Gynecol Oncol, 2007, 105(3): 563-570.
[6]曹泽毅. 妇科常见肿瘤诊治指南(第三版) [M]. 中华妇科肿瘤学分会, 2011, 6: 93-114.
[7]RAGOUSSIS J, SENGER G, TROWSDALE J, et al. Genomic organization of the human folate receptor genes on chromosome 11q13 [J]. Genomics, 1992, 14(2): 423-430.
[8]FIGINI M, FERRI R, MEZZANZANICA D, et al. Reversion of transformed phenotype in ovarian cancer cells by intracellular expression of anti-folate receptor antibodies [J]. Gene Therapy, 2003, l0(12): 1018-1025.
[9]LMA D W, FIRMELL R H, DAVIDSON L A, et al. Folate transport gene inactivation in mice increases sensitivity to colon carcinogenesis [J]. Cancer Res, 2005, 65(3): 887-897.
[10]YUAN Y, NYMOEN D A, DONG H P, et al. Expression of the folate receptor genes FOLR1 and FOLR3 differentiates ovarian carcinoma from breast carcinoma and malignant mesothelioma in serous effusions [J]. Hum Pathol, 2009, 40(10): 1453-1460.
[11]LIU L, ZHU XL, ZHANG D M, et al. An electrochemical method to detect folate receptor positive tumor cells [J].Electrochem Commun, 2007, 9(12): 2547-2550.
[12]TOFFOLI G, CERNIGOI C, RUSSO A, et al. Overexpression of folate binding protein in ovarian cancers [J]. Int J Cancer, 1997, 74(2): 193-198.
[13]KIMBERLY R, KALLI A O, GARY L, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer [J].Gynecol Oncol, 2008, 108(3): 619-626.
[14]ALEXANDRE J, RAY-COQUARD I, SELLE F, et al.Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience [J]. Ann Oncol,2010, 21(12): 2377-2381.
[15]SHEN D W, SU A, LIANG X J,et al. Reduced expression of small GTPases and hypermethylation of the folate binding protein gene in cisplatin-resistant cells [J]. Br J Cancer,2004, 91(2): 270-276.
[16]LIANG X J, SHEN D W, GARFIELD S, et al. Mislocalization of membrane proteins associated with multidrug resistance in cisplatin-resistant cancer cell lines [J]. Cancer Res, 2003,63(18): 5909-5916.
[17]LU Y, LOW P S. Folate-mediated delivery of macromolecular anticancer therapeutic agents [J]. Adv Drug Deliv Rev,2002, 54(5): 675-693.
[18]ROY E J, GAWLICK U, ORR B A, et al. Folate-mediated targeting of T cells to tumors [J]. Adv Drug Deliv Rev,2004, 56(8): 1219-1231.
[19]JEMAL A, SIEGEL R, WARD E, et al. Cancer statistics, 2008[J]. CA Cancer J Clin, 2008, 58(2): 71-96.
[20]TRIMBOS J B, PARMAR M, VERGOTE I, et al. International collaborative ovarian neoplasm trial 1 and adjuvant chemotherapy in ovarian neoplasm trial: two parallel randomized phase Ⅲ trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma [J]. J Natl Cancer Inst, 2003, 95(2): 105-112.
[21]赵群, 段微. CA125阴性卵巢癌血清标志物差异蛋白质组学的研究 [J]. 现代妇产科进展, 2008, 17(5): 337-341.
[22]BASAL E, EGHBALI-FATOURECHI G Z, KALLI K R, et al. Functional folate receptor alpha is elevated in the blood of ovarian cancer patients [J]. PLoS One, 2009, 4(7): 6292.
[23]YAKIREVICH E, SABO E, NARODITSKY I, et a1. Multidrug resistance-related henotype and apoptosis-related protein expression in ovarian serous carcinomas [J]. Gynecol Oncol, 2006, l00(1): 152-159.
Detection of folate binding protein and its clinical significance in ovarian cancers
HUANG Ming-ju,WANG Qi, ZHANG Wei, YIN Qiao-yun, LI Zhuang, LI Li(Department of Gynecologic Oncology,Department of Basic Experiment, Tumor Hospital of Guangxi Medical University, Nanning Guangxi 530021, China)
LI Li E-mail:lili@gxmu.edu.cn
Background and purpose:Folate binding protein (FOLR1), also known as folate receptor protein, plays a key role in division, proliferation and growth of cells. It was less expressed in normal tissues, but over-expression often existed in ovarian cancer. Our study aimed to explore the expression level of FOLR1 and its clinical significance in ovarian cancer.Methods:Western blot was used to detect the expression levels of FOLR1 in 80 ovarian cancer tissues, 50 benign ovarian tumor tissues and 30 normal ovarian tissues, and its association with clinical pathology and multidrug resistance (MDR) of ovarian cancers was analyzed.Results:The expression levels of FOLR1 increased orderly in normal ovarian tissues, benign ovarian tumor tissues and ovarian cancer tissues, and the difference was statistically significant (P<0.01). For ovarian cancer, the expression levels of FOLR1 in clinical stage Ⅰ-Ⅱ were higher than that of stage Ⅲ-Ⅳ, and the difference was statistically significant (P<0.05). For ovarian cancer tissues, the FOLR1 levels of the platinum-resistant were less than the platinum-sensitive, and the difference was statistically significant (P<0.01). After chemotherapy, the FOLR1 levels of progressive ovarian cancer tissues were lower than those in remission, and the difference was statistically significant (P<0.01). For ovarian cancer tissues, the FOLR1 levels of the mucinous type were lower than that of the serous, and the difference was statistically significant(P<0.05). The FOLR1 levels of ovarian cancer with metastasis to lymph nodes and distant organs were higher than those without metastasis, and the difference was statistically significant (P<0.05). However, the FOLR1 levels had no significant association with pathological classification, amounts of ascites and metastasis to the omentum (P>0.05).ROC curve determining the FOLR1 levels associated nature of ovarian tumors demonstrated that the maximum Youden index was 3.115 and the FOLR1 levels showed no obvious correlation with median survival time (P>0.05).COX multivariate analysis denied the FOLR-α level as an independent prognostic factor.Conclusion:The FOLR1 levels of normal ovarian tissues and benign ovarian tumor tissues are lower than that of ovarian cancer tissues, which suggests FOLR1 could be a new biomarker for early diagnosis of ovarian carcinomas. Meanwhile, the FOLR1 levels in ovarian cancer tissues are low for the platinum-resistant but high for the platinum-sensitive, which suggests that FOLR1 is associated with multidrug resistance of ovarian carcinoma and could be a potential target for judging multidrug resistance.
Ovarian cancer; Folate binding protein; Drug resistance
10.3969/j.issn.1007-3969.2012.01.006
R737.31
A
1007-3639(2012)01-0025-06
国家自然科学基金资助项目(No:30960404)。
李力 E-mail:lili@gxmu.edu.cn
2011-08-15
2012-01-01)

