Kinetics of Photocatalytic Degradation of Gaseous Organic Compounds on Modified TiO2/AC Composite Photocatalyst*
YANG Qingshan (杨青山)** , LIAO Yongjin (廖永进) and MAO Lingling (毛玲玲)
1 Guangzhou Unisun Power Technology Co., Ltd., Guangzhou 510600, China
2 Guangdong Power Test and Research Institute, Guangzhou 510600, China
3 Guangdong Inspection & Quarantine Technology Center, Guangzhou 510623, China
1 INTRODUCTION
In recent years, the use of the titanium dioxide(TiO2) as photocatalytic semiconductor material in environmental clean-up operations has aroused great interest due to its non-toxic nature, photochemical stability and low cost, particularly when sunlight is used as the source of irradiation [1-4]. But the shortcomings of conventional TiO2catalyst lie in the low photon utilization efficiency and the difficulty of recovery from reaction medium [5-7]. Therefore, much work has focused on its modification and immobilization [8-13].
Although a great deal of research has been conducted on the photocatalytic reactions, only a few studies have been reported for the photocatalytic degradation kinetics, and the kinetics of photocatalytic oxidation of organic compounds are not clear. However, most researchers believe that the photocatalytic oxidation reaction of organic compounds is a surface reaction, and the rate equation was often expressed by the Langmuir-Hinshelwood (L-H) model as follows [14-16]:

in which c is the concentration of reactants; t is reaction time; k is the rate constant, K is the adsorption constant. The kinetic parameter of k and K are affected by light intensity, temperature, the initial concentration and physical properties of reactants, the gaseous oxygen concentration, etc. In liquid phase reaction system,the initial value of pH is another factor. In suspension system, the geometry and the experiential parameters of the reactors should also be considered [17].
Moreover, the first order kinetic expression has been proved to be compatible for some main factors.Liao et al. investigated the photocatalytic degradation kinetics by changing initial concentrations of formaldehyde on TiO2and confirmed the first order kinetics[18]. By photocatalytic degradation of rhodamine B on TiO2/activated carbon (AC) photocatalyst, Li et al.explored the influence of initial concentration of organic compounds, light intensity and the loading amount of TiO2on reaction kinetics, and the results were showed to be consistent with the first order kinetic model, and the rate constant was obviously dependent on the above factors [19].
In fact, the L-H model is only a kinetic model based on the ideal adsorption, but it is more complicated for actual reaction, especially for gas phase reaction. Moreover, most of study on photocatalytic degradation kinetics paid attention to the effect of a single factor only, meanwhile ignoring the role of multiple factors. In addition, the values of k and K in the L-H model are related with many factors, so it is difficult to use a general mathematical equation to describe the photocatalytic kinetics, and the reaction order may change in different reaction stages. If describing the whole reaction process with a fixed model,significant deviation from the real kinetics would be inevitable [17].
In this article, basing on the previous research results of the photocatalytic degradation of organic compounds in gas phase on modified TiO2/AC composite photocatalyst (MTA) [20], we fit the experimental data to the zero, first and second order kinetic model equations, and the factors that affecting the kinetic model were explored.
2 EXPERIMENTAL
2.1 Photocatalyst preparation
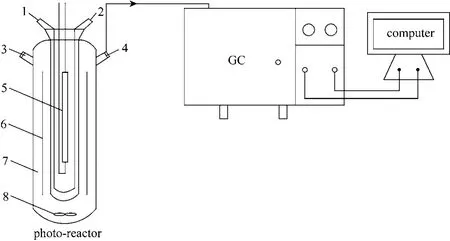
Figure 1 Schematic diagram of photo-reactor and GC analysis1—coolant inlet; 2—coolant outlet; 3—organic feed tap; 4—sampling port; 5—high-pressure mercury lamp; 6—glass plates; 7-reactor; 8—fan
The commercially available AC (AR, Guangdong Taishan Overseas Reagent Plastic Co.) was used, its specific surface area was 1147.5 m2·g-1, and the nominal size was less than 0.18 mm. The MTA was synthesized by the sol-gel method at room temperature as follows: 20 ml butyl titanate (concentration>97%) was slowly dropped into the mixture of 70 ml absolute ethanol and 18 g AC under stirring, adjusted pH=2-3 with acetic acid (HAc), then the suspension was stirred vigorously for 30 min followed by adding a mixture of 60 ml water, 1.012 g ammonium sulfate and 2.065 g ferric nitrate, which was also adjusted pH=2-3 with HAc. The resulted solution was stirred for 2 h, and then stood still for 48 h before drying at 100 °C. MTA was obtained after calcining in stagnant air at 500 °C for 2 h. The modified TiO2composite photocatalyst (MT) was also prepared at the same preparation conditions (without adding AC) [4, 19]. For comparison, P-25 catalyst (product of Degussa, Germany) was used as the photocatalyst directly in photocatalytic reaction.
2.2 Experimental procedure
The uniform mixture of 2 g MTA (MT or P-25)and 10 g water glass (about 25% of SiO2) was coated on one side of four clear glass plates (the total effective illumination area about 331 cm2), drying in a vacuum drier at 100 °C for 2 h, then the four plates were placed into a photo-reactor (SGY-1, Nanjing Sidongke Electric Equipment Co.) as can be seen in Fig. 1, which is a 1200 ml quartz cylindrical vessel with a water-cooled jacket and a fan in the bottom. Certain amount of organic compound was injected into the reactor, which was kept in dark for 60 min to ensure a sufficient volatilization and the establishment of gas adsorption/desorption equilibrium. Irradiation was provided by a 300 W high-pressure mercury lamp (effective arc length 125±10 mm, stabilization time 5 min) with major emission at 365 nm. By sampling (0.1 ml) gaseous mixture from photo-reactor at 20 min intervals, the concentration of organic compounds can be calculated from the analytical results of GC (9800TFP, Shanghai Kechuang Chromatograph Instruments Co., China).
Degradation rate of organic compounds could be evaluated with the following equation:
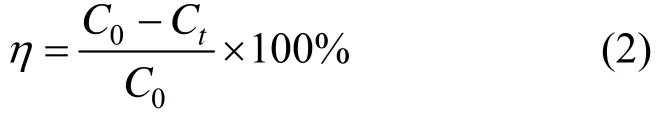
whereC0is the initial concentration of organic compounds when achieving the stabilization time,Ctis the concentration of organic compounds at timet.
3 RESULTS AND DISCUSSION
3.1 Characterization
Figure 2 shows the scanning electron micrograph(SEM) of MTA. It can be seen that part of TiO2(white particles) is wrapped in the surface of AC (gray), and the other part of TiO2is embedded in the hole of AC.The loss of adsorption sites of AC due to impregnation is not significant.

Figure 2 SEM of MTA
Figure 3 shows the UV-visible (UV-Vis) absorption spectra of different catalysts. Comparing the curves 1 and 2 can be found that the absorption spectrum of MT undergoes an obvious red shift, and the absorption intensity in visible region increases, but it weakens in UV region. Comparing the curves 2 and 3 can be seen that MTA shows strong absorption intensity in the UV and visible region.
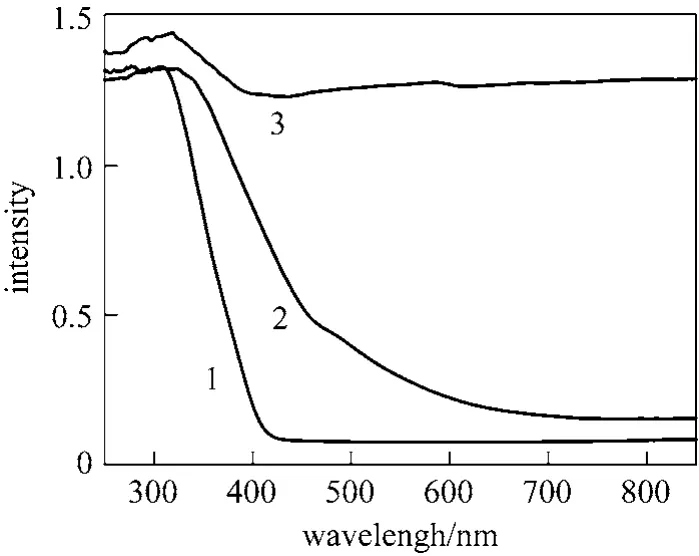
Figure 3 UV-Vis absorption spectra of the catalysts prepared at different conditions1—P-25; 2—MT; 3—MTA
3.2 Performance of photocatalyst
Experiments on photocatalytic degradation of toluene, acetone and formaldehyde vapors were conducted on MTA, and the degradation curves are shown in Fig. 4.

Figure 4 The degradation capability of MTA for different organic compounds(c0 toluene=44.30 g·m-3, c0 acetone=39.40 g·m-3, c0 formaldehyde=39.40 g·m-3)■ toluene; ● formaldehyde; ▲ acetone
As can be seen from Fig. 4, there is little change in degradation rate of toluene and acetone when using MTA, and it maintains a high degradation rate (about 90% in 120 min), but the degradation rate of formaldehyde is lower (only 35% in 120 min).
Experiments on photocatalytic degradation of toluene were also conducted with different initial concentrations: 39.88, 42.05, 43.50, 47.13 g·m-3on MTA, and the results can be seen in Fig. 5. In this range of concentration, the degradation rate of toluene in 120 min increases with the increase of the initial concentration.
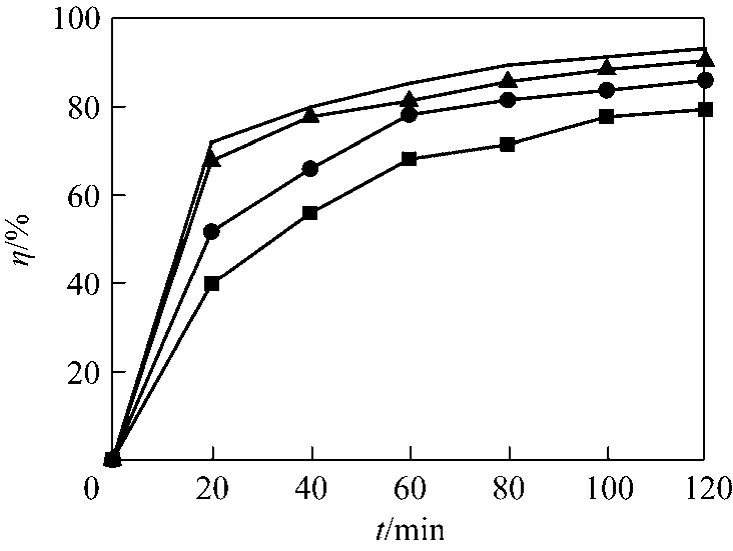
Figure 5 Influence of initial toluene concentrations on degradation rate■ 39.88 g·m-3; ● 42.05 g·m-3; ▲ 43.50 g·m-3; × 47.13 g·m-3
3.3 Kinetic model analysis of photocatalytic reaction
3.3.1Kinetic model of toluene
Figure 6 shows the fitting results of zero, first and second order kinetic models based on the experimental degradation data of toluene in Fig. 4. It can be concluded that the second order kinetic model equation:X/c0(1-X)=ktcorrelates the experimental data quite well, which is different from the first order kinetic model reported in the literature [16, 21]. It is probably because the L-H model is based on the kinetic model of ideal adsorption, but it is more complex for actual kinetics. In particular, the molecular diffusion rate of gas-solid phase photocatalytic reaction is very high, toluene may occur in two different kinds of oxidation under the existence of a large number of electrons and holes [22]. In this study, it is also because of the strong adsorption capacity of MTA,a large amount of toluene was needed in the process of photocatalytic degradation, which leading to a higher concentration of toluene adsorbed on its surface,therefore, the deviation from the application conditions of the L-H model is inevitable. Besides the specific physical properties of black AC can greatly enhance the absorption of light by MTA, which not only affects the stability of photocatalytic temperature, but also increased the probability of directly photolysis for toluene, so the degradation reaction of toluene may be more complicated.
3.3.2Kinetic model of acetone
Table 1 shows the fitting results of the kinetic models basing on the experimental data of acetone in Fig. 4. It is apparent from the results in Table 1 that the data fits well with the second order kinetic model equation, too. The reasons may be related to the same as that of toluene.
3.3.3Kinetic model of formaldehyde
Table 2 shows the fitting results of the kinetic models basing on the experimental data of formaldehyde with initial concentrationc0=39.40 g·m-3in Fig. 4. It can be seen that all the fitting results show a well linear relationship. However, the value of correlation coefficientR2from the zero order kinetic model is higher than others, and the fitting residual of data points is more randomly distributed, suggesting that the photo catalysis is more in line with the zero order kinetic model equation:c0X=kt.

Figure 6 The kinetic model of photocatalytic reaction of toluene
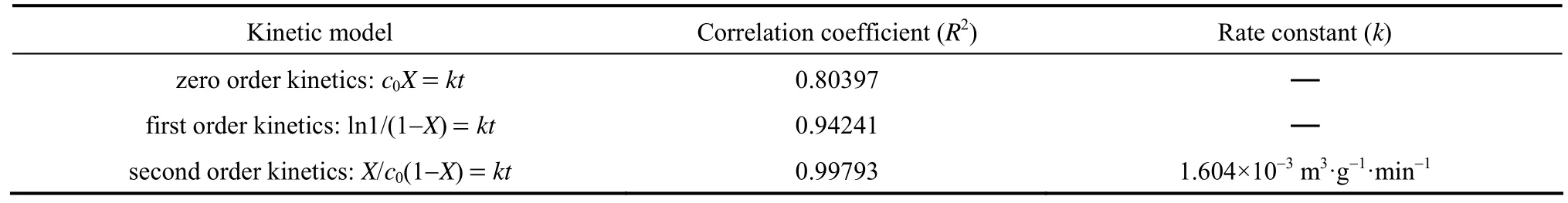
Table 1 The kinetic parameters of photocatalytic degradation of acetone

Table 2 The kinetic parameters of photocatalytic degradation of formaldehyde
The factors affecting the fitting result of kinetic model as follows: because the adsorption properties of AC is strong, and formaldehyde was added into the reactor in form of aqueous solution, the excessive water vapor not only aggravated the competitive adsorption between water molecules and formaldehyde molecules on the surface of MTA, but also hindered the contact of formaldehyde with TiO2[23, 24]. This resulted in the degradation rate mainly controlled by the diffusion rate finally, so that the real kinetics was masked.
3.3.4Effect of initial toluene concentration
Table 3 shows the fitting results basing on the experimental data of different initial toluene concentrations in Fig. 5. It is evidently observed that the fitting results are all fit well with the second order kinetic model equation.
It is apparent from the results in Table 3 that therate constant increases with increasing toluene concentration. This can be explained as follows: The essence of photocatalytic reaction is the indirect photolysis reaction of hydroxyl radicals (OH-) and organic compounds, the molecules of H2O is an important source of OH-, but the amount of catalyst and H2O in air is finite for a closed system. The presence of AC can enhance the adsorption capacity of MTA, and improve the separation efficiency of electrons and holes. When the initial toluene concentration increases, the equilibrium adsorption of the MTA increases, followed by the increase of photocatalytic reaction rate and the amount of H2O (equivalent to improve the air humidity of the system), thereby the content of hydroxyl radicals will be increased under UV irradiation [23].
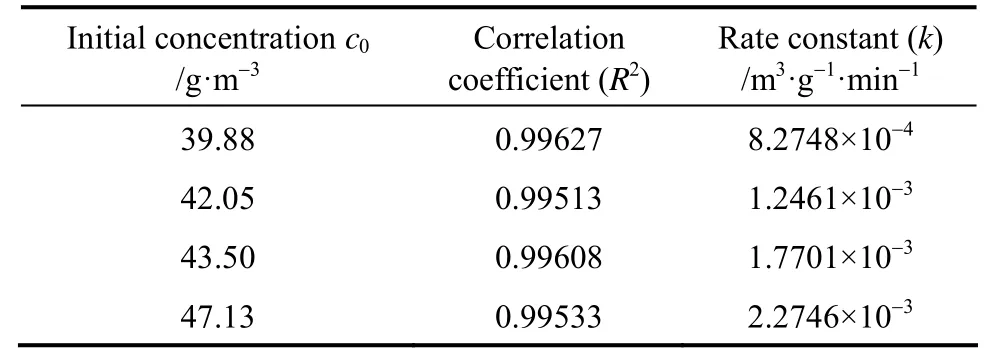
Table 3 The second order kinetic parameters of different initial toluene concentrations
4 CONCLUSIONS
The kinetic models of the photocatalytic degradation of organic compounds by MTA were investigated with respect to the species of organic compounds and different initial toluene concentrations. The fitting results of toluene and acetone were ascertained to fit quite well with the second order kinetic model, and the degradation of formaldehyde fits well with the zero order kinetic model. With higher initial concentration of toluene, the second order kinetic rate constant increased significantly. The validity of the model was partly proved by fitting the experimental data obtained to zero, first and second order kinetic expressions under various conditions.
1 Gaya, U.I., Abdullah, A.H., “Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems”,J.Photochem.Photobiol.C:Photochem.Rev., 9 (1), 1-12 (2008).
2 Tryba B., “Immobilization of TiO2and Fe-C-TiO2photocatalysts on the cotton material for application in a flow photocatalytic reactor for decomposition of phenol in water”,J.Hazard.Mater., 151 (2-3),623-627 (2008).
3 Sun, R.B., Xi, Z.G., Chao, F.H., Zhang, W., Zhang, H.S., Yang, D.F.,“Decomposition of low-concentration gas-phase toluene using plasma-driven photocatalyst reactor”,Atmos.Environ., 41 (32),6853-6859 (2007).
4 El-Bahy, Z.M., Ismail, A.A., Mohamed, R.M., “Enhancement of titania by doping rare earth for photo degradation of organic dye (Direct Blue)”,J.Hazard.Mater., 166 (1), 138-143 (2009).
5 Seery, M.K., George, R., Floris, P., Pillai, S.C., “Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis”,J.Photochem.Photobiol.A, 189 (2-3), 258-263 (2007).
6 Wu, P.X., Tang, J.W., Dang Z., “Preparation and photocatalysis of TiO2nanoparticles doped with nitrogen and cadmium”,Mater.Chem.Phys., 103 (2-3), 264-269 (2007).
7 Zhang, X.W., Zhou, M.H., Lei, L.C., “Preparation of anatase TiO2supported on alumina by different metal organic chemical vapor deposition methods”,Appl.Catal.A, 282 (1-2), 285-293 (2005).
8 Choi, W., Termin, A., Hoffmann, M.R., “The role of metal ion dopants in quantum-sided TiO2: Correlation between photoreactivity and charge carrier recombination dynamics”,J.Phys.Chem., 98 (51),13669-13679 (1994).
9 Sathish, M., Viswanathan, B., Viswanath, R.P., “Characterization and photocatalytic activity of N-doped TiO2prepared by thermal decomposition of Ti-melamine complex”,Appl.Catal.B, 74 (3-4),307-312 (2007).
10 Song, S., Tu, J.J., Xu, L.J., Xu, X., He, Z.Q., Qiu, J.P., Ni, J.G., Chen,J.M., “Preparation of a titanium dioxide photocatalyst codoped with cerium and iodine and its performance in the degradation of oxalic acid”,Chemosphere, 73 (9), 1401-1406 (2008).
11 Mahalakshmi, M., Vishnu Priya, S., Arabindoo, B., Palanichamy, M.,Murugesan, V., “Photocatalytic degradation of aqueous propoxur solution using TiO2and Hβ zeolite-supported TiO2”,J.Hazard.Mater., 161 (1), 336-343 (2009).
12 Plesch, G., Gorbár, M., Vogt, U., Jesenák, K., Vargová, M., “Reticulated macroporous ceramic foam supported TiO2for photocatalytic applications”,Mater.Lett., 63 (3-4), 461-463 (2009).
13 Zhang, X.W., Zhou, M.H., Lei, L.C., “Enhancing the concentration of TiO2photocatalyst on the external surface of activated carbon by MOCVD”,Mater.Res.Bull., 40 (11), 1899-1904 (2005).
14 Takashi, T., Mamoru, F., Tetsuro, M., “Mechanistic insight into the TiO2photocatalytic reactions: Design of new photocatalysts”,J.Phys.Chem.C, 111 (14), 5259-5275 (2007).
15 Maruga, J., Grieken, R.V., Cassano, A.E., Alfano, O.M., “Intrinsic kinetic modeling with explicit radiation absorption effects of the photocatalytic oxidation of cyanide with TiO2and silica-supported TiO2suspensions”,Appl.Catal.,B:Environmental, 85 (1-2), 48-60(2008).
16 Wang, W.Q., Zhang, Q.C., Jian, L., Liu, J.M., Wei, Y.H., “Preparation of regular TiO2-carbide catalyst and its photocatalytic properties”,Journal of Inner Mongolia University of Technology, 26 (4),260-264 (2007). (in Chinese)
17 Dai, D.M., Zhou, J., Gao, H.T., “The advancement on nano-TiO2photocatalytic oxidation technology”,Journal of Liaocheng University(Nat.Sic.), 20 (3), 56-60 (2007). (in Chinese)
18 Liao, D.L., Xiao, X.Y., Deng, Q., Zhang, H.P., Wan, C.X., “Study on kinetics of formaldehyde photocatalytic degradation on titanium dioxide”,Environmental Protectionof Chemical Industry, 23 (4),191-194 (2003). (in Chinese)
19 Li, Y.J., Sun, S.G., Ma, M.Y., Ouyang, Y.Z., Yan, W.B., “Kinetic study and model of the photocatalytic degradation of rhodamine B(RhB) by a TiO2-coated activated carbon catalyst: Effects of initial RhB content, light intensity and TiO2content in the catalyst”,Chem.Eng.J., 142 (2), 147-155 (2008).
20 Yang, Q.S., Liao, Y.J., Xiao, X.Y., “Preparation of iron, nitrogen co-doped TiO2/AC composite photo catalysts and their photo catalytic activities for degradation of gaseous pollutants”,Materials Review, 25 (17), 62-67 (2011). (in Chinese)
21 Yin, Y.Q., Zheng, Y., Su, Y.C., Cui, Q., Cui, Z.J., “Reaction kinetics and mechanism of photocatalytic degradation of gaseous toluene”,Chinese Journal of Process Engineering, 9 (3), 536-540 (2009). (in Chinese)
22 Zhang, J.C., Guo, K.M., Ma, L., Zhao, H.Y., “Reaction mechanisms for gas-phase photocatalytic degradation of benzene and butyraldehyde over TiO2/AC composite photocatalyst”,Chinese Journal of Catalysis, 27 (10), 853-856 (2006). (in Chinese)
23 Guo, T., Bai, Z.P., Wu, C., Zhu, T., “Influence of environmental humidity on the photocatalytic oxidation of toluene by TiO2Loaded on activated carbon fibers”,Chinese Journal of Catalysis, 28 (12),1089-1095 (2007). (in Chinese)
24 Li, B., Li, B.Z., Li, J., Liu, X., “Investigation of the influencing factors on ACF/TiO2photocatalytic degradation of formaldehyde”,Sciencepaper Online, 3 (5), 347-350 (2008). (in Chinese)
 Chinese Journal of Chemical Engineering2012年3期
Chinese Journal of Chemical Engineering2012年3期
- Chinese Journal of Chemical Engineering的其它文章
- Analysis of Absorption and Reaction Kinetics in the Oxidation of Organics in Effluents Using a Porous Electrode Ozonator*
- Flame Imaging in Meso-scale Porous Media Burner Using Electrical Capacitance Tomography*
- Modelling and Fixed Bed Column Adsorption of Cr(VI) onto Orthophosphoric Acid-activated Lignin
- Advances in Large-eddy Simulation of Two-phase Combustion (I)LES of Spray Combustion*
- Top-cited Articles in Chemical Engineering in Science Citation Index Expanded: A Bibliometric Analysis
- Adsorptive Removal of Para-chlorophenol Using Stratified Tapered Activated Carbon Column
