Effect of Polyethylene Glycol on the Properties of γ-Al2O3 Formation by Polyaluminum Chloride*
ZHAO Changwei (赵长伟)**, WANG Xiaonian (王孝年), HE Jingsong (何劲松) WANG Yuanyuan (王园园) and LUAN Zhaokun (栾兆坤)
1 State Key Laboratory of Environmental Aquatic Chemistry, Research Center for Eco-Environmental Sciences,Chinese Academy of Sciences, Beijing 100085, China
2 The Equipment Research Institute of PLA’s Second Artillery, Beijing 100085, China
1 INTRODUCTION
γ-Al2O3is widely used as catalysts, adsorbents,coatings and alumina derived materials in many processes [1-6]. Macro-mesostructured γ-Al2O3support with high BET surface area is important and useful for the treatment of bulky molecules, such as petroleum heavy cuts and residues. In order to prepare macromesostructured γ-Al2O3, using surfactant as pore forming additive is a feasible method to improve surface area of alumina sample [7-10]. Different aluminum sources and preparation methods lead to different sample properties. It is significant to find new source to prepare pseudo-boehmite and γ-Al2O3. Polyaluminium chloride (PAC) is an intermediate product in the processes of hydrolysis, polymerization, gelation and precipitation of aluminum species [11-15]. Among Al species in PAC, active species Al13has an ε-Keggin structure of the tridecameric polymer with a charge of+7 and a size of 1.06 nm, and is now used in many fields such as catalysis, material and environment protection for its nano-sized and positively charged characteristics [16-19].
Previous study proved that PAC including Al13species can be used as raw material to prepare pseudo-boehmite and γ-Al2O3[20, 21]. However, there are few reports on surfactant effect on sample property using new PAC including Al13species as aluminum source. The goal of this work is the study of the influence of polyethylene glycol (PEG) with different molecular mass on pseudo-boehmite and γ-Al2O3when high concentration PAC including high Al13content is as new aluminum source.
2 EXPERIMENTAL
2.1 Materials and method
In order to investigate the property and broaden the application of a new high concentration PAC prepared from our laboratory, a 2.1 mol·L-1concentration PAC solution self-made by chemical synthesis-membrane distillation (MD) method: Firstly, a 0.2 mol·L-1PAC was prepared by neutralizing 170 ml of 1 mol·L-1AlCl3aqueous solution in a 1000-ml glass reactor kept at 65 °C by adding 420 ml of 0.6 mol·L-1NaOH solution and reacting for 12 h at 65 °C. Then MD process was applied to concentrate the 0.2 mol·L-1PAC solution in a membrane module equipped with flat-sheet membrane having efficient area of 70 cm2. The membrane material is a hydrophobic microporous PVDF(IPVH00010, Millipore) with 0.45 μm pore size and is placed between the two identical chambers. The temperature of the feed and the permeate were controlled at 55 °C and 20 °C respectively. The permeate side was flushed with tap water. After MD of 30 h, 480 ml of 2.1 mol·L-1concentration PAC solution was prepared and used as raw material for preparing spherical γ-Al2O3.
NH3·H2O (purity>99%), nitric acid (purity>99%),liquid paraffin (purity>99%), PEG 1000, 4000 and 10000 (purity>99%) were supplied from Beijing Chemical Reagent Company, Beijing, China.
Ferron reagent (mixture reagent) was prepared as follows [22]: 0.2% (by mass) Ferron (8-hydroxy-7-iodoquinoline-5-sulfonic acid, Sigma Chemical Co.,USA) solution, 20% NaAc solution, and 100 ml was mixed with 900 ml of deionized water and 1∶9 HCl(using 37% hydrochloric acid) solution were prepared and filtered through pre-washed 0.45 μm membranes separately. The Ferron colorimetric solution was obtained by mixing 0.2% Ferron, 20% NaAc and 1∶9 HCl at a ratio of 2.5∶2∶1.
2.2 Preparation of spherical pseudo-boehmite and γ-Al2O3
Spherical pseudo-boehmite and its derivative γ-Al2O3were prepared by a combination of the sol-gel method and oil-drop method. Pseudo-boehmite sol was firstly prepared by sol-gel method as follows:70 ml of 4% NH3·H2O solution was added into 250 ml of 2.1 mol·L-1PAC solution in a bath temperature of 65 °C, then PEG 1000, 4000 or 10000 used as surfactant separately was added into solution up to the content range of 5%-25% under mechanical stirring of 40 min at the same temperature. The resulted white slurry was aged for 6 h to form alumina hydrate gel and then washed with distilled water to pH value to 7. After that,1 mol·L-1HNO3was peptized into gel at the H+/Al3+molar ratio of 0.12 under ultrasonic action for 20 min.The resulted pseudo-alumina sol became viscous and was transferred to a dropper for granulation process. The droplets first fell through liquid paraffin layer (relative density 0.845-0.890 g·cm-3, viscosity 30.42-32.04 mPa·s) and partially formed spherical wet-gel granules by the surface tension, and then the wet-gel granules fell through an 8% (by mass) ammonia solution layer with the 45 cm depth. The structure of the wet-gel granules were consolidated after aging in the ammonia solution for 4 h. The granules were then removed from the ammonia solution, dried in an ambient oven at 60 °C for 12 h, and finally calcined in the muffle furnace at 450 °C for 8 h.
2.3 Analytical methods
The Al species in solution was measured by the Al-Ferron Assay [23] with a transient colorimetric reaction with Ferron reagent to provide Al species distribution based on chemical reactivity on a UV-Vis spectrophotometer (DR/4000U, Hach, USA). Based on the kinetic difference of the reactions between the Al species and Ferron reagent, the hydrolyzed Al species can be divided into three types: monomeric species (Ala) (reacting with Ferron from 0 min to 1 min),oligomeric and medium polymeric species (Alb) (reacting with Ferron from 1 min to 120 min), and colloidal hydroxides (Alc) (reacting with Ferron after 120 min and non-reacting with Ferron).27Al solution nuclear magnetic resonance (NMR) spectroscopy was used as another analytical tool to characterize the aluminum species distribution.27Al NMR spectra were generated at 70 °C on a Fast Fourier Transformation spectrometer (JNM-ECA600, JOEL, Japan), which was operated at a field strength of 14.09 T, single pulse method, resonating frequency 156.39 MHz,X-pulse of 6.4 μs, X-acq-duration 0.20 s, repetition time 0.50 s, relaxation delay 0.30 s, scans 2048.Transmission electron microscopic (TEM) image was obtained on a TECNOL 20 microscope (Philips, Dutch).The crystalline phases of the samples were characterized by X-ray diffraction (Shimadzu XRD-6000, Japan) technique at 40 kV and 30 mA, with Cu Kαradiation,λ=0.15418 nm. Diffraction patterns were run from 10°-80° at a scan speed of 5°·min-1. Nitrogen adsorption-desorption isotherms (ASAP 2000, Micrometrics, USA) were determined and the surface areas were calculated using the BET equation. The pore volume and pore size distribution were determined by mercury porosimetry (ASAP 2000, Micromeritics,USA). The spectrum of the samples was obtained by Fourier transform infrared spectroscopy (FT-IR) (Nexus 670, Nicolet, USA) at room temperature using KBr pellets. Thermogravimetric-differential thermal analysis (TG-DTA) curves of the samples were carried out using a TGA-Q50 (TA-Waters, USA) instrument at a heating rate of 10 °C·min-1.
3 RESULTS AND DISCUSSION
3.1 Al species distribution
The 2.1 mol·L-1PAC with high Al13content of 81.5% (based on moles of Al) was prepared by chemical synthesis-membrane distillation method. The content of Al species in the solutions is shown in Table 1.

Table 1 Al species distribution of PAC solutions by ferron assay
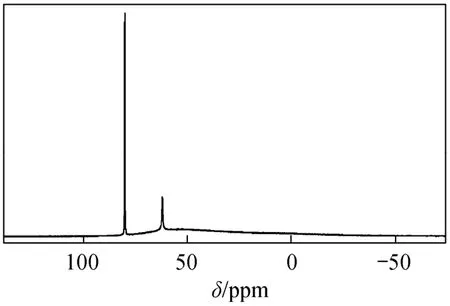
Figure 1 27Al NMR spectrum of a polyaluminum chloride solution
Figure 1 shows the27Al NMR spectrum signals of PAC solution sample with high Al13content in our laboratory. The peak at 63.0 ppm can be attributed to central tetrahedral Al atom of the Keggin[AlO4Al12(OH)24(H2O)12]7+ion Al13. Firstly, The 80 ppm resonance represented the standard sample, sodium aluminate, and was set as 1.0. Integrating 0 ppm peak area, a standard curve was obtained regarding the peak area as abscissa and Al concentration as the coordinate. The aluminum content was calculated according to the standard curve with the 80 ppm peak area as the basis. The Al13polymerization percentage was obtained as central tetrahedral Al atom of the Keggin [AlO4Al12(OH)24(H2O)12]7+ion multiplied by 13.
3.2 Effect of PEG with different molecular mass on γ-Al2O3 sample
In experiment, PEG 1000, 4000 and 10000 were chosen as surfactant and added with the 15% content to PAC solution. The effects of PEG with different molecular mass on γ-Al2O3sample were investigated.The surface area, pore volume and pore size of γ-Al2O3after 450 °C calcination with different PEG as surfactant are shown in Table 2.
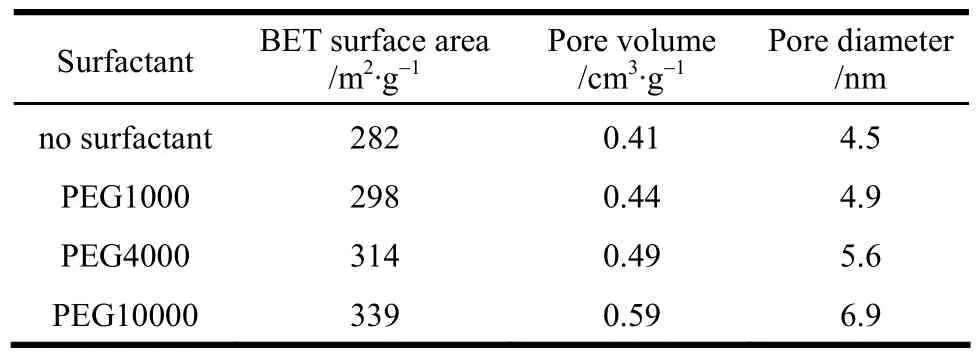
Table 2 Pore structure of γ-Al2O3 granules obtained with different surfactants① Experimental conditions: PEG addition is 15%, calcined at 450 °C.
From Table 2 we can see that surface area, pore volume and pore size of γ-Al2O3all increased with the increase of PEG molecular mass in the experimental range. PEG 10000 is most suitable for increasing surface area of γ-Al2O3. Based on PEG10000 as surfactant, Table 3 gives the effects of surfactant content on γ-Al2O3sample calcined at 450 °C. It can be seen that the surface area, pore volume and pore size are the biggest at PEG10000 15% in the experimental range.
Figure 2 shows the micro-structure of γ-Al2O3after 450 °C calcination with different PEG as surfactant at 15% content. From TEM images it can be seen that the structure are porous for every sample, pore diameter of the sample with PEG 10000 is the largest.

Figure 2 TEM images of γ-Al2O3 granules obtained with different surfactants

Table 3 Pore structure of γ-Al2O3 granules obtained with different PEG10000 content
3.3 Effect of PEG with different molecular mass on alumina sample phase
To further characterize alumina crystal morphology, the XRD patterns of alumina samples with different PEG as surfactants are shown in Fig. 3. The diffraction pattern of every sample after 450 °C calcination shows the main strong diffraction peaks of γ-Al2O3at 2θ=19.27°, 37.43°, 39.66°, 46.02°, 66.73°.It can be seen the sample can retain its structure although surfactant added. The result indicated that PAC with high Al13content can be used as raw material to prepare the spherical γ-Al2O3granules by sol-gel method.
3.4 Pore forming mechanism of PEG with different molecular mass
To further investigate the pore forming mechanism of PEG 1000, 4000 and 10000 as surfactant, the FT-IR spectra is characterized and shown in Fig. 4,showing that the peaks at 1384 cm-1, 1070 cm-1and 842-420 cm-1can be attributed to the pseudo-boehmite.The adsorption peak at 1633 cm-1is due to the complex peak of the residual water intercalated in the layers of pseudo-boehmite and OH groups on the surface of pseudo-boehmite. Compared to curve without surfactant, the curve with surfactant shows distinct peaks located around 2900 cm-1and 952 cm-1. These peaks are due to the vibration of OH group and C O C group of PEG. The results illustrate that PEG molecules are chemisorbed on the surface of the products.

Figure 3 XRD patterns of γ-Al2O3 granules obtained with different surfactants
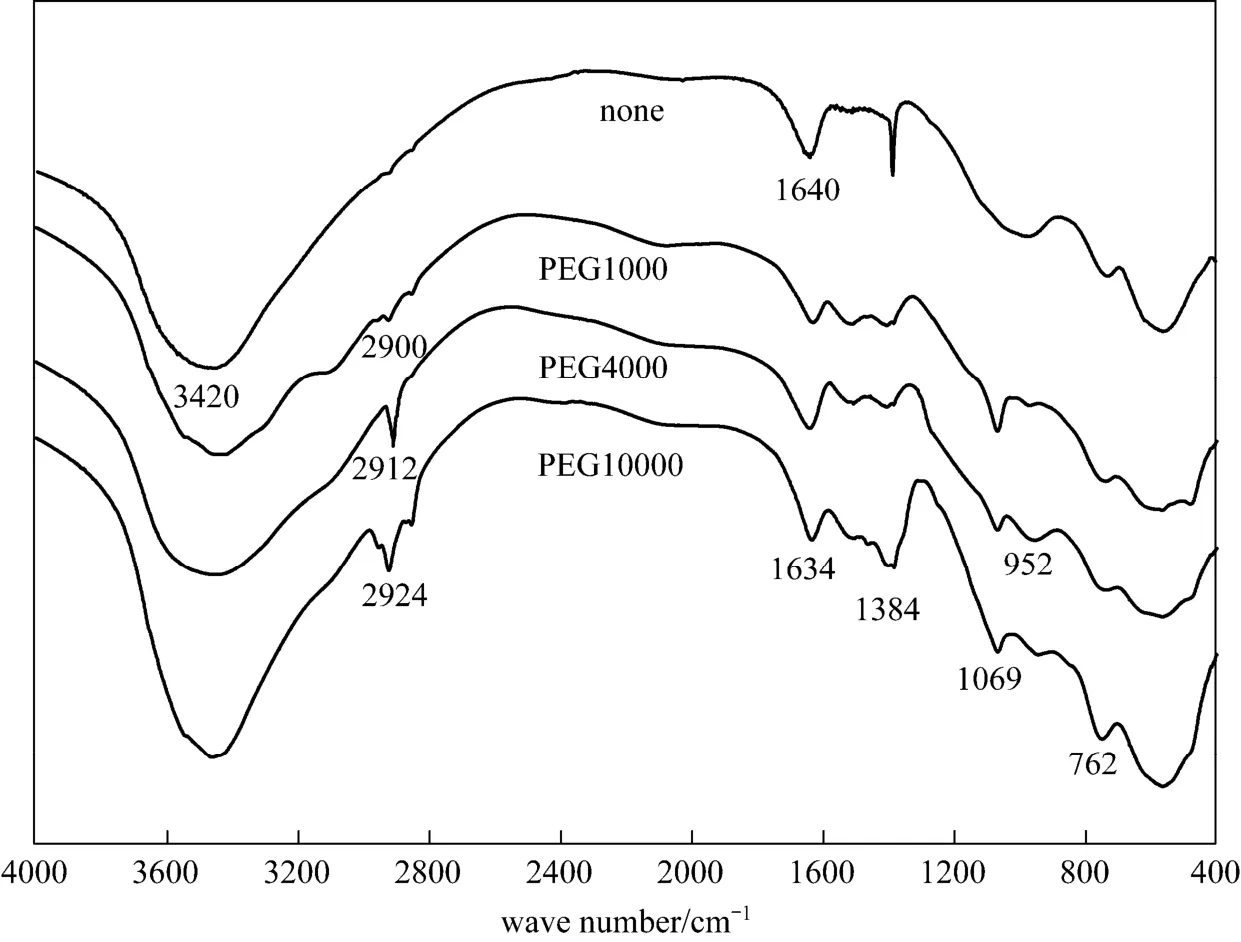
Figure 4 FT-IR spectra of spherical pseudo-boehmite granules with different surfactants
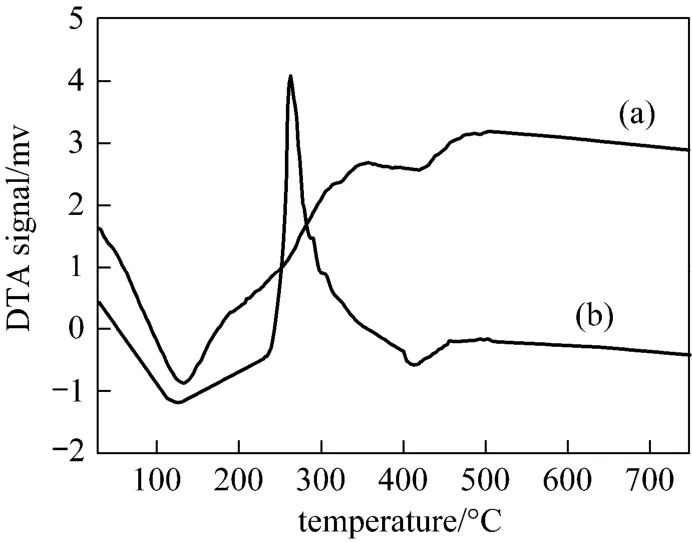
Figure 5 DTA curve of pseudo-boehmite granules (a) no surfactant; (b) PEG 10000 surfactant
Figure 5 illustrates the TG-DTA results of samples with and without PEG10000 surfactant. From Fig. 5 (a)we can see that the DTA curve show that two endothermic peaks exist at around 132 °C and 418 °C. There is also one broad exothermic peak corresponding to the second mass loss process. An endothermic peak at 132°C is due to desorption of the surface water. The endothermic peak at 418 °C is attributed to the release of interlayer water. There is no distinct peak after 450 °C in the range of experimental research temperature,which indicates that pseudo-boehmite transformed completely to γ-Al2O3. It is thus known that the excess water molecules in the colloid were removed by calcination. As shown in Fig. 5 (b) with PEG 10000 surfactant, the DTA curve show that one endothermic peak exists between 135 and 145 °C, which is result of release of desorption of the surface water and PEG evaporation. Around 262 °C there is one exothermic peak corresponding to the second mass loss process,which is result of decomposition of PEG 10000 surfactant. There is also no distinct peak after 450 °C in the range of experimental research temperature, which indicates that pseudo-boehmite transformed to γ-Al2O3.
1 Zhang, X., Zhang, F., Chan, K.Y., “The synthesis of large mesopores alumina by microemulsion templating, their characterization and properties as catalyst support”, Mater. Lett., 58, 2872-2877 (2004).
2 Cai, W.Q., Yu, X.F., “Preparation of macro-mesostructured pseudoboehmite and γ-Al2O3with high surface area”, Progr. Chem., 19,1322-1330 (2007). (in Chinese)
3 Ren, T.Z., Yuan, Z.Y., Su, B.L., “Microwave-assisted preparation of hierarchical mesoporous-macroporous boehmite AlOOH and γ-Al2O3”,Langmuir, 20, 1531-1534 (2004).
4 Rachiero, G.P., Demirci, U.B., Miele, P., “Facile systhesis by polyol method of a ruthenium catalyst supported on γ-Al2O3for hydrolytic dehydrogenation of ammonia borane”, Catal. Today, 170, 85-92 (2011).5 Chen, R.Z., Du, Y., Xing, W.H., “Effect of alumina particle size on Ni/Al2O3catalysts for p-nitrophenol hydrogenation”, Chin. J. Chem.Eng., 15 (6), 884-888 (2007).
6 Dang, D., Ding, W.M., Ceng, A., “Isotherm equation study of F adsorbed from water solution by Fe2(SO4)3-modified granular activated alumina”, Chin. J. Chem. Eng., 19 (4), 581-585 (2011).
7 Liu, M.Z, Yang, H.M., “Large surface area mesoporous Al2O3from kaolin: Methodology and characterization”, Appl. Clay. Sci., 50,554-559 (2010).
8 Gonzalez-Pena, V., Diaz, I., Marquez-Alvarez, C., “Thermally stable mesoporous alumina synthesized with non-ionic surfactants in the presence of amines”, Micro. Mes. Mater., 44-45, 203-210 (2001).
9 Zhang, Z.R., Pinnavaia, T.J., “Mesostructured γ-Al2O3with a lathlike framework morphology”, J. Am. Chem. Soc., 124, 12294-12301 (2002).
10 Cai, W.Q., Li, H.Q., Zhang, Y., “Azeotropic distillation-assisted preparation of macro-mesostructured γ-Al2O3nanofibres of crumpled sheet-like morphology”, Mater. Chem. Phys., 96, 136-139 (2006).
11 Luan, Z.K., “Study on basic theory and application of inorganic polymer flocculate polyaluminum chloride”, Ph.D. Thesis, Research Center for Eco-Environmental Science, CAS, China (1997). (in Chinese)
12 Qu, J.H., Liu, H.J., “Optimum conditions for Al13polymer formation in PACl preparation by electrolysis process”, Chemosphere, 55,51-56 (2004).
13 Hu, C.Z., Liu, H.J., Qu, J.H., “Coagulation behavior of aluminum salts in eutrophic water: significance of Al13species and pH control”,Environ. Sci. Technol., 40, 325-331 (2006).
14 Gao, B.Y., Chu, Y. B., Yue, Q.Y., “Characterization and coagulation of a polyaluminum chloride (PAC) coagulant with high Al13content”,J. Environ. Sci., 21, 18-22 (2005). (in Chinese)
15 Lin, J.L., Huang, C.H., Pan, J.R., “Effect of Al(III) speciation on coagulation of highly turbid water”, Chemosphere, 72, 189-196(2008).
16 Sanabria, N.R., Centeno, M.A., Molina, R., “Pillared clays with Al-Fe and Al-Ce-Fe in concentrated medium: Synthesis and catalytic activity”, Appl. Catal. A Gen., 356, 243-249 (2009).
17 Vicente, M.A., Belver, C., Trujillano, R., “Preparation and characterisation of vanadium catalysts supported over alumina-pillared clays”,Catal. Today, 78, 181-190 (2003).
18 Casey, W.H., “Large aqueous aluminum hydroxide molecules”,Chem. Rev., 106, 1-16 (2006).
19 Qian, Z.S., Feng, H., Yang, W.J., “Theoretical investigation of water exchange on the nanorneter-sized polyoxocation A lO4A l12( OH)24( H2O )172+(Keggin-Al13) in aqueous solution”, J. Am. Chem. Soc., 130,14402-14403 (2008).
20 Zhao, C.W., He, J.S., Ren, X.J., “Preparation and property of pseudoboehmite by polyaluminum chloride”, Chin. Inorg. Chem., 26,521-524 (2010). (in Chinese)
21 Zhao, C.W., Wang, X. N., Luan, Z.K., “Preparation and characterization of γ-Al2O3by polyaluminum chloride with high Al13content”,Chin. J. Chem. Eng., 18 (2), 333-336 (2010).
22 Feng, C.H., Shi, B.Y., Wang, D.S., “Characteristics of simplified ferron colorimetric solution and its application in hydroxy-aluminum speciation”, Colloids Surfaces A Physicochem. Eng. Aspects., 287,203-211 (2006).
23 Chen, Z.Y., Fan, B., Peng, X.J., “Evaluation of Al30polynuclear species in polyaluminum solutions as coagulant for water treatment”, Chemosphere, 64, 912-918 (2006).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- Direct Synthesis of Dimethyl Carbonate from CO2 and CH3OH Using 0.4 nm Molecular Sieve Supported Cu-Ni Bimetal Catalyst*
- Measurements of Conductivity for Low Concentration Strongelectrolytes in Organic Solvents (I) LiBr, LiCl, and LiNO3 in Alcohols
- CO2 Capture by Vacuum Swing Adsorption Using F200 and Sorbead WS as Protective Pre-layers*
