Dose requirement and complications of diluted and undiluted propofol for deep sedation in endoscopic retrograde cholangiopancreatography
Somchai Amornyotin, Wichit Srikureja, Wiyada Chalayonnavin and Siriporn Kongphlay
Bangkok, Thailand
Dose requirement and complications of diluted and undiluted propofol for deep sedation in endoscopic retrograde cholangiopancreatography
Somchai Amornyotin, Wichit Srikureja, Wiyada Chalayonnavin and Siriporn Kongphlay
Bangkok, Thailand
(Heaobiliary Pancreat Dis Int 2011; 10: 313-318)
propofol;dose requirement;complication;endoscopic retrograde cholangiopancreatography
Introduction
There is a growing interest in using propofol for sedation in endoscopic procedures.[1-3]Propofol is commonly used as an anesthetic agent in endoscopic retrograde cholangiopancreatography (ERCP).It is a fast-acting drug with a short half-life that results in rapid recovery. It also has a favorable pharmacokinetic pro fi le suitable for induction and maintaining intravenous anesthesia. However, it has a narrow therapeutic window and has cardiorespiratory depressant effects. Many previous studies have reported that a slower rate of propofol administration for anesthesia results in smaller dose requirements.[4,5]Hypotension during induction is reported to be attenuated by the use of diluted propofol.[6]
Currently, administration of propofol is guided by monitoring of clinical signs and hemodynamic data.However, the level of consciousness cannot be reliably judged by somatic or hemodynamic responses alone.A reliable method is needed to measure the hypnotic component of sedation and anesthesia. Recently,processed electroencephalogram (EEG) variables such as the spectral edge frequency, the bispectral index (BIS) or NarcotrendTMwere developed to ease EEG interpretation.These tools have been reported to be precise in the measurement of sedation level.[7-9]
NarcotrendTM(Monitor Technik, Bad Bramstedt,Germany) was developed by a research group atHannover University Medical School, Germany. It performs a computerized analysis of the raw EEG. Two EEG channels are recorded, comparing signals from the two hemispheres of the brain. A multivariate statistical algorithm is used for analysis which results in a six-stage classi fi cation from A (awake) to F (general anesthesia/coma) and 14 substages.[10]The newest NarcotrendTMsystem additionally includes a dimensionless Narcotrend index from 100 (awake) to 0 (electrical silence), similar to the BIS.
Our previous study showed that dose requirement and sedation-related complications using diluted propofol for sedation in patients undergoing ERCP were signi fi cantly lower than using undiluted propofol.However, patients in that study were sedated using only clinical assessment.[11]Therefore, our aim in this study was to compare the clinical ef fi cacy of diluted and undiluted propofol using the Narcotrend index as guide for depth of anesthesia in patients undergoing ERCP.
MethodsPatients
A total of 86 consecutive patients from a tertiary care teaching hospital, Siriraj Hospital, Bangkok, Thailand,were eligible for the study. These patients were at least 18 years of age and underwent ERCP between February 2005 and January 2006. Exclusion criteria included any clinical evidence of severe liver disease(hepatic encephalopathy, cirrhosis with marked ascites),American Society of Anesthesiologists (ASA) physical status IV or V, and patients who had severe hypovolemia(systolic blood pressure <80 mmHg) at the time of presentation for the procedure. This present study was approved by the Institutional Review Board of the Faculty of Medicine, Siriraj Hospital. All the enrolled patients provided written informed consent to undergo the procedures and to participate in the study.
Study design
This study was a randomized controlled study. The primary outcome variable was the total dose of propofol used during the procedure. The secondary outcome variables were complications during and immediately after the procedure and recovery time. The amount of propofol used was compared as total dose, dose/kg and dose/kg per hour. Recovery time was de fi ned as the time after completion of the procedure to the awakening of the patient.
All patients were randomized into either the diluted propofol group (group D) or the standard/undiluted propofol group (group U) by using sealed envelopes.Forty-four patients were randomized to group D whereas 42 patients were randomized to group U.Standard propofol was diluted with normal saline in a 1∶1 ratio by a researcher who did not sedate the patient.The dose used in group D was 5 mg/mL and in group U was 10 mg/mL. All patients were monitored in the standard fashion. In addition, the depth of sedation was monitored using the NarcotrendTMsystem. The anesthetic personnel who sedated the patient were blinded not only to the randomization process but also to the concentration of propofol used for sedation.The blinding to the concentration of the propofol was possible because both concentrations appeared similar and were delivered in similar syringes and volumes.
ERCP procedure
The procedure was done using an Olympus video duodenoscope (TJF 160 R, Olympus Corp., Tokyo,Japan) by three staff endoscopists, who had more than ten years of ERCP experience. The procedure was performed with the patient either in the prone or left lateral position.
Sedation procedure
All sedation was carried out by the anesthetic personnel, who were either anesthetic nurses or secondyear residents in the anesthesiology residency program,directly supervised by a staff anesthesiologist in the endoscopy room. Each patient was monitored for blood pressure, heart rate, electrocardiogram and oxygen saturation. No premedications were administered before the procedure. All patients received supplemental oxygenation via a nasal cannula.
The patients were sedated with 0.02-0.03 mg/kg midazolam (total dose ≤2 mg for age <70 years and 1 mg for age ≥70) and 0.5-1 μg/kg fentanyl (total dose≤75 μg for age <70 and ≤50 μg for age ≥70) as well as propofol with a dose concentration depending on the randomization procedure. All sedations were deep,in accordance with the guidelines of the American Society of Anesthesiologists.[12]In both groups, diluted or undiluted, propofol was titrated continuously using the NarcotrendTMsystem as guide for depth of sedation.Propofol was given intravenously by continuous infusion with a syringe pump in all patients. Five minutes before the end of the procedure, continuous intravenous infusion of propofol was stopped. Crystalloid solution was used for maintenance fl uid in all cases. The total amount of intravenous fl uid including crystalloid solution and normal saline used in both groups were comparable.
Sedation level assessment
The NarcotrendTMsystem classi fi cation of depth of sedation is in six stages from A (awake) to F (general anesthesia/coma) and 14 substages. All sedations were maintained with the NarcotrendTMsystem at stage D0(index 57-64). If the depth of sedation was too light, a bolus dose of propofol (10-20 mg) was administered until the target Narcotrend stage was reached. In addition,if the patient was sedated too deeply, the intravenous infusion of propofol was temporarily stopped.
Propofol requirement and complications
The amount of propofol used (mean total dose,dose/kg, and dose/kg per hour) was compared between the two groups. In addition, the recovery time of both groups was evaluated. The complications during and immediately after ERCP were recorded: hypotension(decrease by 20% from baseline or systolic blood pressure <90 mmHg), bradycardia (decrease in heart rate by 20% from baseline or heart rate <50 beats/min),as well as oxygen desaturation (SpO2<90%) and upper airway obstruction.
Statistical analysis
The study was designed to test the null hypothesis that the propofol requirement in the diluted propofol group was lower than that in the undiluted propofol group using the NarcotrendTMsystem. As was reported this monitoring potentially reduced the dose of sedative agents used and complications and ensured a rapid recovery time.[13-15]We hypothesized that the propofol requirement was about 50% with the undiluted propofol in contrast to 20% with the diluted propofol. To detect a difference in the propofol requirement between the groups, the estimated sample size was 40 patients per arm. The power of the test was 0.8. In addition, α was set to 0.05 for all comparisons. Results were expressed as mean±SD or percentage (%) when appropriate. The statistical software package SPSS for Windows Version 11.0 (SPSS Inc., Chicago, IL) was used to analyze the data.A signi fi cance level of 5% was used throughout the study.
Results
The clinical characteristics, duration of sedation and indications of procedure of all the patients are summarized in Table 1. The mean ages in groups D and U were 57.7±16.1 and 58.8±13.7 years, respectively.Patients in both groups were similar with respect to age, gender, weight, height, ASA physical status, preprocedure volume status, pre-anesthetic problems,duration of sedation and indications for ERCP. All procedures were completed successfully using the intravenous sedation (IVS) technique.
The mean dose of propofol used and recovery time categorized by age in both groups are listed in Table 2.The mean total dose, dose/kg and dose/kg per hour of propofol in both groups were not signi fi cantly different.In addition, the mean recovery time among patients in groups D and U was not signi fi cantly different.
The Narcotrend index and the hemodynamic parameters (systolic and diastolic blood pressure), heart rate as well as oxygen saturation are shown in Table 3.The mean Narcotrend index and mean systolic bloodpressure throughout the study were not signi fi cantly different between the two groups. In addition, the mean diastolic blood pressure was not signi fi cantly different between the two groups except at scope insertion. At this time, the mean diastolic blood pressure was slightly lower in group U than that in group D. However, the reduction of mean diastolic blood pressure was stillwithin the satisfactory range, and this was not clinically signi fi cant. Moreover, there were no signi fi cant differences in heart rate and SpO2throughout the study.Mean SpO2of all patients was 99% throughout the procedure.
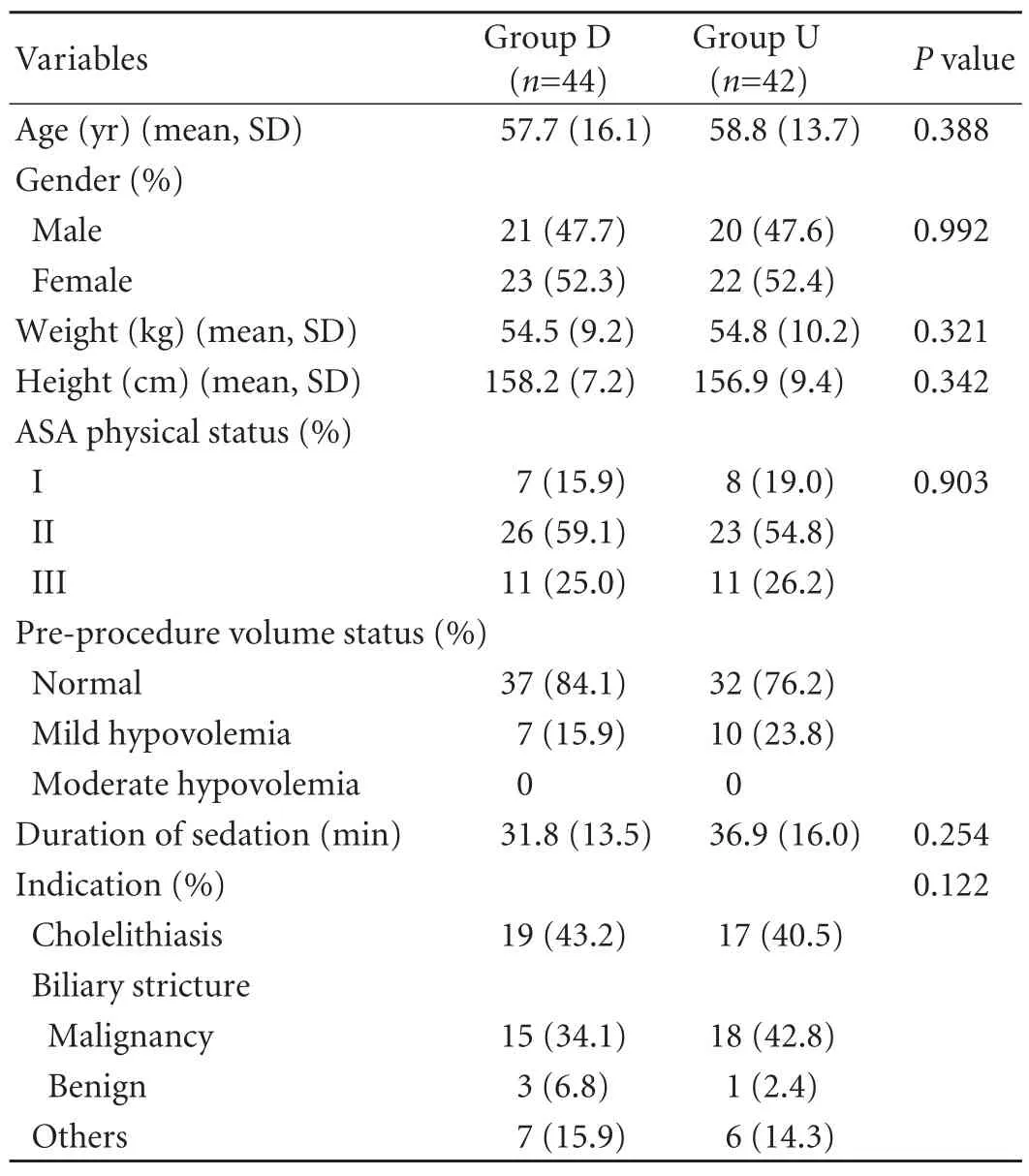
Table 1. Characteristics of patients, duration of sedation and indications of procedure

Table 2. Mean dose of propofol and recovery time (mean±SD;range)

Table 3. Narcotrend index and hemodynamic variables: systolic and diastolic blood pressure (mmHg), heart rate (beats/min) and oxygen saturation (%) (mean±SD).

Table 4. Sedation-related adverse events during and immediately after ERCP (n, %)
Overall sedation-related adverse events during and immediately after ERCP in both groups are shown in Table 4. Patients in group D had signi fi cantly lower overall complications than those in group U. In addition, cardiovascular-related adverse events in group D were also signi fi cantly lower than those in group U.Hypotension was the most common adverse event in both groups. However, signi fi cantly less hypotension was observed in group D than in group U. One patient each in groups D and U developed hypotension and bradycardia. In addition, no respiratory adverse events were noted in group D. All patients who had complications in both groups did not differ in ASA physical status, duration of sedation and type of intervention. Moreover, all complications were minor and easily treated without sequelae.
Discussion
This present clinical study compared the dose requirement, recovery time, and complications in the use of diluted and undiluted propofol as guided by NarcotrendTMsystem monitoring in patients who underwent intravenous sedation for ERCP. Our results demonstrated that propofol in both forms can be successfully used to provide deep sedation in patients undergoing ERCP without serious adverse events.The use of diluted propofol was shown to be more advantageous as it signi fi cantly reduced sedation-related adverse events.
EEG-based monitoring was fi rst introduced with BIS monitoring in 1996. The number shown on the monitor is processed from the EEG and correlates well with the level of sedation. The NarcotrendTMsystem is similarly EEG-guided. Previous studies[13-15]showed a suf fi cient correlation between Narcotrend and BIS.The Narcotrend scale is divided into 14 substages (A,B0-2, C0-2, D0-2, E0, 1 and F0, 1) with an additional index from 100 (awake) to 0 (no cortical activity).The correlation between Narcotrend stages and the respective Narcotrend index ranges was described by Kreuer et al.[14]
Moderate or deep sedation is often used for patients undergoing ERCP. The current practice in our hospital is that all sedation for ERCP procedures is done using propofol administered by the anesthesiology team.Clinical assessment alone has been the guide for depth of sedation in titrating propofol dose. Our practices do not routinely use EEG-base monitoring devices.
Serious complications with propofol-based sedation,especially respiratory and cardiovascular adverse events,can occur. Although rare, these complications need to be rapidly recognized and appropriately managed to avoid the risk of brain damage, cardiac arrest, or death.Given its narrow therapeutic window and short halflife, propofol needs to be carefully titrated to effect.Careful monitoring of depth of sedation, therefore,is important. In previous studies, there were large variations in the dosages of propofol given for sedation during endoscopic procedures.[2,3]Consequently, there was a great individual variability in drug levels needed to achieve certain desired effects. There is no absolute correlation between administered doses of sedatives and the level of responsiveness. Monitoring of the depth of sedation is therefore important.
Traditional methods for assessing the level of sedation have relied primarily on subjective assessment of the patient and alteration in vital signs. However, the value of hemodynamics to assess the depth of anesthesia remains controversial.[16]Mean arterial pressure can only be an indirect parameter to estimate hypnotic effects.Given these limitations, monitors that can objectively assess the level of sedation would be extremely useful.EEG-based monitoring of the level of consciousness, like BIS or Narcotrend, has added to the armamentarium of tools for monitoring the patient undergoing moderate to deep sedation.
At present, EEG-guided sedation is used by anesthesiologists to achieve exact titration of hypnotic agents.[13,17,18]These monitoring devices are potentially cost-saving in that they may reduce the dose of sedative agents used and allow a rapid recovery time. The impact of EEG-based monitoring in total intravenous anesthesia has been studied. Narcotrend or BIS monitoring in patients undergoing procedures with total intravenous propofol-based anesthesia leads to lower propofol consumption, quicker emergence from anesthesia,earlier extubation, and shorter recovery time.[9,10,15,19]
In our previous study, the use of undiluted propofol was compared with that of diluted propofol during ERCP where only clinical assessment was used to monitor the depth of sedation. Our results showed that patients who received undiluted propofol required higher doses and experienced more complications than those who received diluted propofol.[11]The mechanism of the reduction of propofol requirement in the diluted propofol group remained unclear. We could not explain it from the pharmacokinetic standpoint. However, the difference of propofol requirement was not signi fi cant between the diluted group and the undiluted group in the present study. Surprisingly, in the present study using Narcotrend monitoring, the propofol requirement in both groups was comparable. However, there were similar results that the diluted propofol group demonstrated lower sedation-related complications.
The lack of observance of reduction in propofol consumption and recovery time in our study may be due to the induction regimen used. Even though all patients were sedated with propofol, they were all given an induction regimen with fentanyl and midazolam based on their weight. It is possible that these induction regimens may have equalized the need for propofol between the groups and masked the true impact of EEG-based monitoring on propofol consumption. It is logical that, since there was no difference in the propofol consumption, the study also did not fi nd differences in the recovery time. Again, the recovery time may have been impacted by the administration of fentanyl and midazolam.
The fi ndings are interesting and the mechanism by which the diluted propofol is associated with a lower complication rate during the ERCP procedure is not clear. Fluid replacement during the procedure was comparable in the two groups. Consequently,whether the use of diluted propofol for gastrointestinal procedures will translate to patient tolerance and satisfaction is also not known. The current study did not evaluate cost-effectiveness strategy. However, it is conceivable that the use of diluted propofol with EEG-based monitoring for gastrointestinal endoscopic procedures may be cost-saving, given the lower complication rate.
There are several limitations in our study. Prior to administration of propofol, an induction regimen consisting of fentanyl and midazolam was given to all patients. This may have affected the amount of propofol used, recovery time and perhaps adverse events. Interindividual differences in pharmacodynamics and pharmacokinetics can cause huge inter-individual variance, which may also have affected the results.Last, when assessing the bene fi ts of diluted propofol, a further study with a larger cohort is needed to validate our fi ndings, assess the impact of diluted propofol on patient tolerance and satisfaction, and assess its costeffectiveness especially in light of the additional cost of EEG-based monitors.
In conclusion, the use of NarcotrendTMsystem monitoring in patients undergoing deep sedation with diluted propofol for ERCP did not help to reduce the dose requirement of propofol or shorten the recovery time in contrast to undiluted propofol. However, the sedation-related hypotension in the group with diluted propofol was signi fi cantly lower than that in the group with undiluted propofol.
Funding: None.
Ethical approval: This study was approved by the Institutional Review Board of the Faculty of Medicine, Siriraj Hospital.
Contributors: AS proposed the study and wrote the fi rst draft. AS and SW analyzed the data and wrote the second draft. AS, CW and KS performed the study. All authors contributed to the design and interpretation of the study and to further drafts. AS is the guarantor.Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Krugliak P, Ziff B, Rusabrov Y, Rosenthal A, Fich A, Gurman GM. Propofol versus midazolam for conscious sedation guided by processed EEG during endoscopic retrograde cho langiopancreatography: a prospective, randomized, doubleblind study. Endoscopy 2000;32:677-682.
2 Jung M, Hofmann C, Kiesslich R, Brackertz A. Improved sedation in diagnostic and therapeutic ERCP: propofol is an alternative to midazolam. Endoscopy 2000;32:233-238.
3 Wehrmann T, Kokabpick S, Lembcke B, Caspary WF, Seifert H. Ef fi cacy and safety of intravenous propofol sedation during routine ERCP: a prospective, controlled study. Gastrointest Endosc 1999;49:677-683.
4 Stokes DN, Hutton P. Rate-dependent induction phenomena with propofol: implications for the relative potency of intravenous anesthetics. Anesth Analg 1991;72:578-583.
5 Peacock JE, Lewis RP, Reilly CS, Nimmo WS. Effect of different rates of infusion of propofol for induction of anaesthesia in elderly patients. Br J Anaesth 1990;65:346-352.
6 Kazama T, Ikeda K, Morita K, Kikura M, Ikeda T, Kurita T, et al. Investigation of effective anesthesia induction doses using a wide range of infusion rates with undiluted and diluted propofol. Anesthesiology 2000;92:1017-1028.
7 Schmidt GN, Bischoff P, Standl T, Voigt M, Papavero L,Schulte am Esch J. Narcotrend, bispectral index, and classical electroencephalogram variables during emergence from propofol/remifentanil anesthesia. Anesth Analg 2002;95:1324-1330.
8 Kreuer S, Wilhelm W, Grundmann U, Larsen R, Bruhn J. Narcotrend index versus bispectral index as electroencephalogram measures of anesthetic drug effect during propofol anesthesia. Anesth Analg 2004;98:692-697.
9 Wehrmann T, Grotkamp J, Stergiou N, Riphaus A, Kluge A,Lembcke B, et al. Electroencephalogram monitoring facilitates sedation with propofol for routine ERCP: a randomized,controlled trial. Gastrointest Endosc 2002;56:817-824.
10 Wilhelm W, Kreuer S, Larsen R; Narcotrend-Studiengruppe.Narcotrend EEG monitoring during total intravenous anaesthesia in 4630 patients. Anaesthesist 2002;51:980-988.
11 Amornyotin S, Suraseranivongse S, Muangman S, Sattawatthamrong Y, Tensit K, Prakotsue K, et al. Comparison of dose requirement of diluted and undiluted propofol for patients undergoing ERCP. Thai J Anesthesiol 2003;29:6-12.
12 American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists.Anesthesiology 2002;96:1004-1017.
13 Kreuer S, Biedler A, Larsen R, Schoth S, Altmann S, Wilhelm W. The Narcotrend--a new EEG monitor designed to measure the depth of anaesthesia. A comparison with bispectral index monitoring during propofol-remifentanil-anaesthesia.Anaesthesist 2001;50:921-925.
14 Kreuer S, Bruhn J, Larsen R, Bialas P, Wilhelm W.Comparability of Narcotrend index and bispectral index during propofol anaesthesia. Br J Anaesth 2004;93:235-240.
15 Kreuer S, Biedler A, Larsen R, Altmann S, Wilhelm W.Narcotrend monitoring allows faster emergence and a reduction of drug consumption in propofol-remifentanil anesthesia. Anesthesiology 2003;99:34-41.
16 Struys MM, Jensen EW, Smith W, Smith NT, Rampil I, Dumortier FJ, et al. Performance of the ARX-derived auditory evoked potential index as an indicator of anesthetic depth: a comparison with bispectral index and hemodynamic measures during propofol administration. Anesthesiology 2002;96:803-816.
17 Heier T, Steen PA. Assessment of anaesthesia depth. Acta Anaesthesiol Scand 1996;40:1087-1100.
18 Smith WD, Dutton RC, Smith NT. Measuring the performance of anesthetic depth indicators. Anesthesiology 1996;84:38-51.
19 Weber F, Pohl F, Hollnberger H, Taeger K. Impact of the Narcotrend Index on propofol consumption and emergence times during total intravenous anaesthesia with propofol and remifentanil in children: a clinical utility study. Eur J Anaesthesiol 2005;22:741-747.
BACKGROUND: In general, the dose requirement and complications of propofol are lower when used in the diluted form than in the undiluted form. The aim of this study was to determine the dose requirement and complications of diluted and undiluted propofol for deep sedation in endoscopic retrograde cholangiopancreatography.
METHODS: Eighty-six patients were randomly assigned to either group D (diluted propofol) or U (undiluted propofol). All patients were sedated with 0.02-0.03 mg/kg midazolam (total dose ≤2 mg for age <70 years and 1 mg for age ≥70) and 0.5-1 μg/kg fentanyl (total dose ≤75 μg for age <70 and ≤50 μg for age ≥70). Patients in group U (42) were sedated with standard undiluted propofol (10 mg/mL). Patients in group D (44) were sedated with diluted propofol (5 mg/mL). All patients in both groups were monitored for the depth of sedation using the Narcotrend system. The primary outcome variable was the total dose of propofol used during the procedure. The secondary outcome variables were complications during and immediately after the procedure, and recovery time.
RESULTS: All endoscopies were completed successfully. Mean propofol doses per body weight and per body weight per hour in groups D and U were 3.0 mg/kg, 6.2 mg/kg per hour and 4.7 mg/kg, 8.0 mg/kg per hour, respectively. The mean dose of propofol, expressed as total dose, dose/kg or dose/kg per hour and the recovery time were not signi fi cantly different between the two groups. Sedation-related adverse events during and immediately after the procedure were higher in group U (42.9%)than in group D (18.2%) (P=0.013).CONCLUSIONS: Propofol requirement and recovery time in the diluted and undiluted propofol groups were comparable.However, the sedation-related hypotension was signi fi cantly lower in the diluted group than the undiluted group.
Author Af fi liations: Department of Anesthesiology and Siriraj GI Endoscopy Center, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand (Amornyotin S, Chalayonnavin W and Kongphlay S);Section of Gastroenterology, Loma Linda University Medical Center, Loma Linda, CA, USA (Srikureja W)
Somchai Amornyotin, MD, Department of Anesthesiology and Siriraj GI Endoscopy Center, Faculty of Medicine,Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand (Tel: +662-4197990; Fax: +662-4113256; Email: sisam@mahidol.ac.th)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
December 13, 2010
Accepted after revision February 11, 2011
 Hepatobiliary & Pancreatic Diseases International2011年3期
Hepatobiliary & Pancreatic Diseases International2011年3期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Borderline resectable pancreatic tumors: Is there a need for further re fi nement of this stage?
- Clinical features and treatment of sump syndrome following hepaticojejunostomy
- Current surgical management of pancreatic endocrine tumor liver metastases
- Liver transplantation for hepatocellular carcinoma:an update
- Cytokine and apoptosis gene polymorphisms influence the outcome of hepatitis C virus infection
- Predictive value and main determinants of abnormal features of intraoperative cholangiography during cholecystectomy
