最小相对表观弥散系数值在低级别胶质瘤与反应性胶质增生鉴别诊断中的价值
胡步宏 贾中正 耿道颖
低级别胶质瘤(low-grade glioma,LGG)与反应性胶质增生(reactive gliosis,RG)影像特征相似,在常规CT/MRI上很难鉴别,而两者的治疗方案明显不同。LGG容易恶变成间变性胶质瘤,甚至是胶质母细胞瘤。一旦诊断为LGG,必须尽早手术切除。RG是胶质细胞在数量上的增加,很少恶变(即使恶变成胶质瘤,也需要很长时间),所以RG只需要随访,不需要手术。因此,LGG与RG的准确鉴别对于患者的治疗计划与预后评估异常重要。近年来有关胶质瘤表观弥散系数(ADC)值的研究报道越来越多。因此,我们设想ADC值在鉴别LGG与RG方面可能存在价值。本研究目的是探讨最小相对ADC(rADCmin)值在LGG与RG鉴别诊断中的价值。
方 法
1.临床资料
收集经病理证实的58例LGG(WHO Ⅱ级)和11例RG患者,其中男40例,女29例,年龄10~69岁,平均36.2岁。在MRI检查前所有患者未进行过干预治疗,每例患者均在MRI检查后一周内手术。
2.MRI成像与后处理
所有MRI检查均在GE3.0 M R扫描仪 (Signa VH/I,GE Healthcare,Milwaukee)上进行,扫描使用8通道头颈线圈。常规MRI包括FLAIR(TR/T E/T I 8502ms/124ms/2250ms,层厚 5mm,间隔 2mm,矩阵288×224,FOV24.0×24.0cm,2次采集);T1-FLAIR(T R/TE/T I2205ms/18.3ms/860ms,层厚5mm,间隔 2mm,矩阵 320×224,FOV24cm×18cm,2次采集);T2WI(T R/TE,3600/114ms,层厚5mm,间隔2mm,矩阵416×224,FOV24cm×18cm,2次采集);T1-FLAIR增强与弥散加权成像(DWI)。DWI使用自旋回波平面序列,(T R/T E4800ms/73.4ms,层厚5mm,间隔2mm,矩阵 128×128,FOV24cm×24cm,2次采集,b值为0和1000s/mm2)。
两名经验丰富的放射科医师手工设置感兴趣区,在ADC图中最低信号区测量病变的ADCmin值,测量三次,取平均值。rADCmin值为病变的ADCmin值与对侧正常脑白质ADC值的比值。
3.统计学处理
采用SPSS16.0 统计软件进行数据处理,所有计量资料用表示。采用Mann-Whitney检验分析LGG与RG之间的差异性;检验水准以P<0.05为差异有统计学意义。ROC曲线分析用于诊断LGG的临界值、敏感性和特异性。
结 果
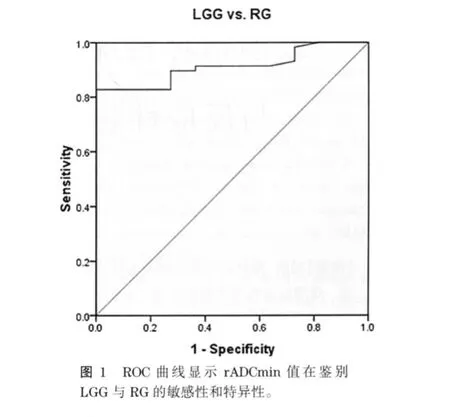
LGG的平均rADCmin为1.465±0.357,高于RG(1.062±0.120)(P<0.05)。ROC曲线下面积为0.912,当临界值rADCmin=1.193时,诊断LGG的敏感性为 82.8%,特异性为100%(图 1)。
讨 论
胶质瘤是中枢神经系统最常见的肿瘤,其中LGG肿瘤细胞分化良好,伴有较多的胶质纤维,并含有较多的微囊变。LGG的常规CT/MRI缺乏特异性,很容易与RG、不典型脑梗死、脑炎和脱髓鞘病变混淆[1-2];尤其是RG,与LGG的临床和影像特征非常相似。RG是在感染、中毒、外伤、缺血缺氧、辐射等因素的刺激下发生的胶质细胞增生,属良性病变[3-7]。RG在病理上主要表现为胶质细胞的增生、变性伴胶质瘢痕形成,常伴有各种炎性细胞浸润。影像表现缺乏特异性,与LGG相似,两者的鉴别很难[8-11],最终需病理确诊。但是两者的生物学行为差异很大,治疗方案也明显不同。
近年来,DWI与ADC值在中枢神经系统的应用越来越广泛。ADC值是通过DWI计算得来的变量参数,利用细胞外水分子运动(布朗运动)的高斯分布特性进行成像,能够提供正常脑与脑病变的解剖和生理信息[12-13]。研究已发现,ADCmin值在胶质瘤与淋巴瘤、脑转移瘤、各种囊性病变的鉴别诊断中具有重要价值[14-17],同时ADCmin值也可以区分胶质瘤的各种成分,包括肿瘤实质、囊变与坏死、瘤周水肿[18-20]。但是有关RG ADC值的报道很少,Hagen等[21]研究发现ADC值在区分血管源性水肿与RG方面存在价值,说明ADCmin值在区分细胞成分与囊性成分方面存在优势。研究发现,细胞数量增加或体积增大,可提高细胞密度,使细胞外水分子的弥散空间减小,弥散受限,ADC值将减低[22-23]。本研究中,LGG的rADCmin值(1.465±0.357)大于 RG(1.062±0.120)(图 2,3),说明LGG与RG尽管都有细胞密度增高,但两者之间还是存在着差别,很可能是病理上的细微差别;LGG在病理上不同于RG的主要差别之一就是LGG存在较多的微囊变,细胞外空间增大,这很可能引起水分子弥散能力增强,引起ADC值的升高。有人研究发现由于高级别胶质瘤(high-grade glioma,HGG)实质部分的ADC值低于LGG实质部分的ADC值,就是因为前者的细胞密度大,细胞外空间减小,水分子弥散受限,所以利用ADC值与胶质瘤分级存在的负性关系,可以对胶质瘤的术前分级提供一定的参考价值[24-27]。同样,淋巴瘤和髓母细胞瘤的细胞密度大,其ADC值远小于胶质瘤[14,28]。已经有报道,可以通过测量胶质瘤瘤周水肿的ADC值来研究颅内肿瘤的鉴别诊断,但目前存在争议[15,20,29]。所以ADC值在中枢神经系统疾病的诊断与鉴别诊断中扮演着相当重要的角色。本研究的限制:第一,由于胶质瘤有时成分不均匀,可以同时含有不同级别的成分,而用于分级的组织标本不一定就是rADCmin对应的肿瘤部分,容易造成分级偏差。第二,DWI技术以水分子的高斯分布为前提,不能真正反映细胞膜、大分子等脑内微结构的复杂性。
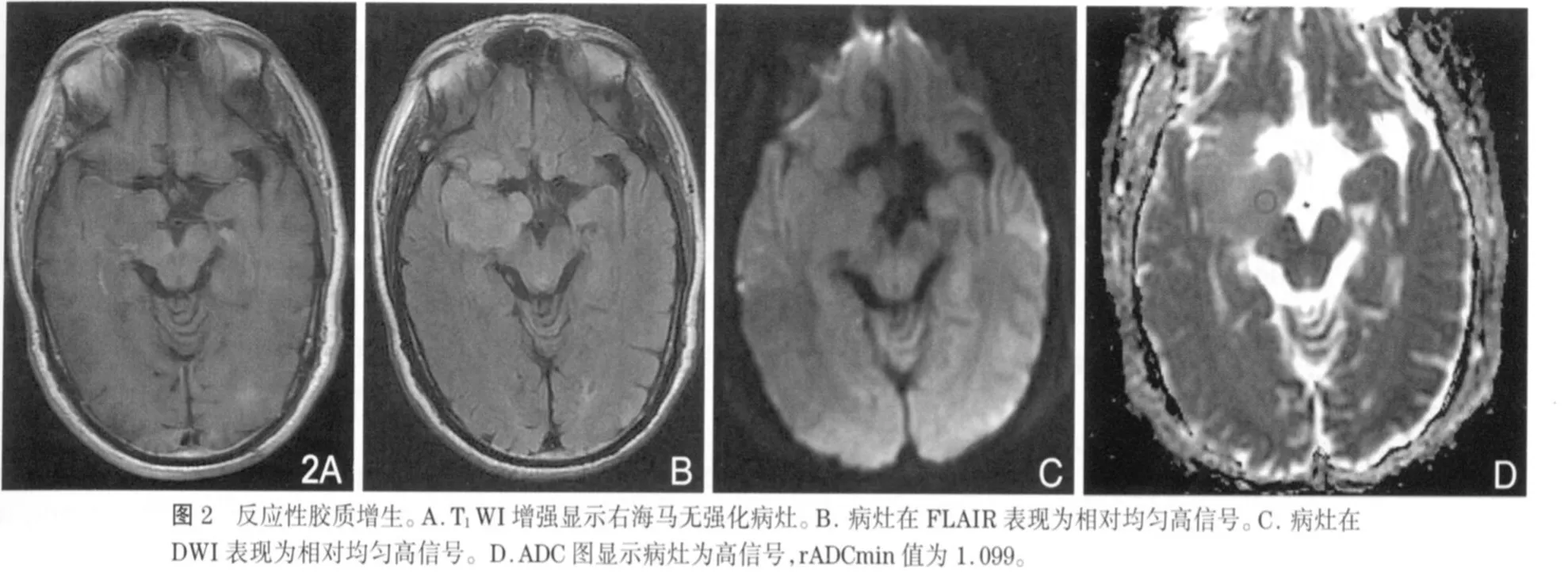
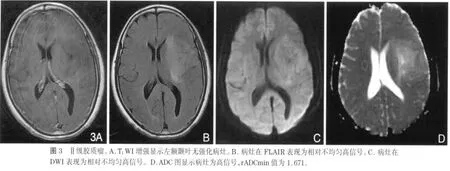
总之,由于rADCmin能够反映LGG与RG在病理组成上的细微差别,所以rADCmin值在LGG与RG的鉴别诊断中能够提供重要的临床价值,有利于降低误诊率,提高患者的预后评估。
1.Pui MH,WangY.Diffusion and magnetization transfer MRI of brain infarct,infection,and tumor in children.Clin Imaging,2005,29:162-171
2.Giussani C,Poliakov A,Ferri RT,et al.DTI fiber trackingto differentiate demyelinatingdiseases from diffuse brain stem glioma.Neuroimage,2010,52:217-223
3.Heindel WC,Jernigan TL,ArchibaldSL,et al.The relationship ofquantitative brain magnetic resonance imagingmeasures to neuropathologic indexes of human immunodeficiency virus infection.Arch Neurol,1994,51:1129-1135
4.McMillian MK,Thai L,HongJS,et al.Brain injuryin a dish:a model for reactivegliosis.Trends Neurosci,1994,17:138-142
5.Faulkner JR,Herrmann JE,Woo MJ,et al.Reactive astrocytes protect tissue andpreserve function after spinal cord injury.J Neurosci,2004,24:2143-2155
6.Wasserman JK,Yang H,Schlichter LC,et al.Glial responses,neuron deathand lesion resolution after intracerebral hemorrhage in young vs.aged rats.Eur J Neurosci,2008,28:1316-1328
7.Hwang SY,Jung JS,Kim TH,et al.Ionizing radiation induces astrocyte gliosis through microglia activation.Neurobiol Dis,2006,21:457-467
8.Cazenave C,Reid S,Virapongse C,et al.Reactive gliosis simulating butterfly glioma:a neuroradiological case study.Neurosurgery,1986,19:816-819
9.Mautner VF,Pressler M,Funsterer C,et al.Cerebral MRT in neurofibromatosis:gliosis versus neoplasia?Rofo,1989,151:225-228
10.Rogers LR,Weinstein MA,Estes ML,et al.Diffuse bilateral cerebral astrocytomas with atypical neuroimagingstudies.J Neurosurg,1994,81:817-821
11.Nishioka H,ItoH,Miki T.Difficulties in the antemortem diagnosisofgliomatosiscerebri:report of a case withdiffuse increase of gemistocyte-like cells,mimicking reactive gliosis.Br J Neurosurg,1996,10:103-107
12.Beaulieu C.The basis of anisotropic water diffusion in the nervous system-a technical review.NMR Biomed,2002,15:435-455
13.Chenevert TL,Sundgren PC,Ross BD.Diffusion imaging:insight to cell status and cytoarchitecture.NeuroimagingClin N Am,2006,16:619-632,viii-ix
14.Horger M,Fenchel M,Nagele T,et al.Water diffusivity:comparison ofprimaryCNS lymphoma and astrocytic tumor infiltratingthe corpus callosum.AJR,2009,193:1384-1387
15.Server A,Kulle B,Maehlen J,et al.Quantitative apparent diffusion coefficients in the characterization of brain tumors and associatedperitumoral edema.Acta Radiol,2009,50:682-689
16.Reiche W,Schuchardt V,Hagen T,et al.Differential diagnosis of intracranial ring enhancing cystic mass lesions--role of diffusion-weighted imaging(DWI)and diffusion-tensor imaging(DTI).Clin Neurol Neurosurg,2010,112:218-225
17.Murakami R,Hirai T,Sugahara T,et al.Grading astrocytic tumors byusingapparent diffusion coefficientparameters:superiorityof a one-versus two-parameterpilot method.Radiology,2009,251:838-845
18.Pavlisa G,Rados M,Pavlisa,G,et al.The differences of water diffusion between brain tissue infiltrated bytumor andperitumoral vasogenic edema.Clin Imaging,2009,33:96-101
19.Li M,Jia ZZ,Gu HM,et al.plication of apparent diffusion coefficient and fractional anisotropyin identification of tumor component andgradingof brain astrocytoma.Zhonghua Yi Xue Za Zhi,2008,88:3352-3355
20.Guzman R,Altrichter S,El-KoussyM,et al.Contribution of the apparent diffusion coefficient inperilesional edema for the assessment of brain tumors.J Neuroradiol,2008,35:224-229
21.Hagen T,Ahlhelm F,Reiche W.Apparent diffusion coefficient in vasogenic edema and reactive astrogliosis.Neuroradiology,2007,49:921-926
22.Yamashita Y,Kumabe T,Higano S,et al.Minimum apparent diffusion coefficient is significantlycorrelated with cellularityin medulloblastomas.Neurol Res,2009.,31:940-946
23.Jenkinson MD,du Plessis DG,Smith TS,et al.Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterizedby genotype.J Neurooncol,2010,96:385-392
24.Arvinda HR,Kesavadas C,Sarma PS,et al.Glioma grading:sensitivity,specificity,positive andnegative predictive values of diffusion andperfusion imaging.J Neurooncol,2009,94:87-96
25.Alvarez-Linera J,Benito-Leon J,Escribano J,et al.Predicting the histopathological grade of cerebral gliomas using high b value MR DW imaging at 3-tesla.J Neuroimaging,2008,18:276-281
26.Higano S,Yun X,Kumabe T,et al.Malignant astrocytic tumors:clinical importance of apparent diffusion coefficient in prediction ofgrade andprognosis.Radiology,2006,241:839-846
27.Miura S,Kurita T,Noda,K,et al.Gradingastrocytic tumors by using apparent diffusion coefficient parameters:superiority of a one-versus two-parameter pilot method.Radiology,2009,251:838-845
28.Rumboldt Z,Camacho DL,Lake D,et al.Apparent diffusion coefficients for differentiation of cerebellar tumors in children.AJNR,2006,27:1362-1369
29.Oh J,Cha S,Aiken AH,et al.Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema.J Magn Reson Imaging,2005,21:701-708

