实验小鼠种群管理
Sonja T.Chou
(上海查士睿华生物医药科技有限公司,上海 201203)
1 Rep roductive Per form ance of M ice
Rodent reproduction is a complex process subject to biological and environmental influences.There are many textbooks that provide information on the general biology and reproductive physiology of the laboratory m ice.A lthough variability can exist between different mouse strains and stocks,and sometimes unpredictable in geneticallymodified mousemodels,such information can and should serve as a baseline with which to establish one’s breeding management practice.Any mouse user should have a good understanding of basic rodent biology and physiology,as well as know ledge regarding rodent genetics in order to successfully manage the colony while meeting production and scientific goals.Two suggested references include an article by Murray and Parker[1],which contained examp les and tips for troubleshooting reproductive performances of mice,and the book chapter by Pritchett and Taft on the reproductive biology of the laboratory mouse,which provided comprehensive coverage on the topic[2].
Breeding systems fall under two general categories.First is the permanently mated groups,which include monogamous and harem breeding.Second,the temporary mated groups,which include polygamous and timed-matings,where the females are separated from the males at some time post mating.Advantages and disadvantages associated with each system are discussed by Murray and Parker[1].To maximize the productivity of femalem ice,they are best kept in permanently mated groups to take advantage of the post-partum estrus.Monogamous breeding cages will result in the most number of pups born per female over her reproductive lifespan.On the other hand,havingmultiple females in the same cage(i.e.harem breeding)will result in the most number of pups born per breeder cage,although inadvertently individual productivity will decrease. To maximize the productivity by a single male mouse,itmay be best to rotate different receptive females to the stud cage on a weekly basis.
Mating scheme refers to breeder set-up based on genetic background and zygosity of the breeders;for example,mating of a heterozygous male to a homozygous female. When breeding genetically modified rodents,the mating scheme will depend on the phenotype of the model,the production goals,and in some cases,limited by the availability of the breeders.To m inim ize breeding errors,mouse users should be required to have a good understanding of basic principles of genetics.
2 M aintenance of Breed ing Records
Keeping organized breeding records is an essential part of production colonymanagement.It allows one to generate reproductive performance data that are reflective of one’s own production colony,as well as help one establish realistic expectations and troubleshoot production.
Basic breeding information can easily be tracked at cage side.For example,on the cage card one can list the identification and date of birth of the breeders,date of breeding unit set-up,age of the breeders at first litter,the dates of all litters born,number of pups born and weaned in each litter,sex distribution within each litter,and expected retirement age/date of the breeders.The retained information can further be used to assess the reproductive performance of a particular colony.For example,the frequency with which each mating pair or unit will produce offspring(e.g.productive mating frequency),the age at which the female will first become pregnant,typical litter size,interval between litters,cumulative number of litters and/or offspring,and the average number of litters that a single female can produce over her life time.
Suggested reading on systems for keeping breeding colony records include the chapter by W.White on management and design of breeding facilities[3],the chapter by Festing and Peters on animal production and breeding methods[4],and text by L.Silver on mouse genetics[5].
3 Breeder Selection and Rep lacem ent
Keep in mind that a mouse colony consists of three functional categories:breeders,future breeders,and stock.Active breeders produce stock animals that are used on studies,but a certain number ofmice need to be set aside as future breeders to rep lace a portion of current breeding pool as their reproductive performance declines.In general,select animals that exhibit the normal characteristics for that strain or stock as future breeders.When maintaining inbred colonies,choose sibling pairs from parents that exhibit the standard phenotype reported.Make sure a breeder rotation schedule is established in order to sustain the colony size while meeting short and long term production goals.The breeder rotation schedule will depend on the effective reproductive lifespan of the animalmodel.For example,if the effective reproductive lifespan of your breeder is roughly 5 months,then it is recommended that 20%of the existing older breeders be retired and rep laced every month.If the effective reproductive lifespan is 10 months,then 10%of the breeding pool should be rotated off every month[6].
Mostm ice should be producing a litter every 3 to 4 weeks,with a visible decrease in reproductive performance as they age.Mating cages that are exhibiting poor reproductive performance and not meeting expectations should be investigated,and possibly replaced.For example,mating cages that do not produce any litters after 2 to 3 months after set-up,lack of new litters produced after 2 months,or consecutive loss of 3 litters due to inability for a cage to raise or wean pups.Mice that exhibit health issues or have anatomical deform ities are not likely to be effective breeders.This includes certain diseasemodels that are genetically predisposed to shortened lifespan or sub-optimal parenting abilities,and timely replacement of breeders or setting up alternative breeding schemes should be used.
Through periodic review of production data,a good colony manager should be able to further characterize the reproductive performance and lifespan of a particular model,as well as effectively troubleshoot production problems and implement appropriate corrective measures.
4 Troub leshooting Production
Many factors related to the animals and their environments are known to influence the reproductive performance of mice,and should be considered when troubleshooting production problems.For a more detailed list and in depth review of these factors,p lease see the chapter by Pritchett and Taft[1].Historic data have shown that the reproductive performance ofmice varied by strains and stocks,and these data can be found in various texts[3,5].Specific strain characteristics or mutations can also impact the parenting ability of m ice.Biological problems that influence production can include a combination of age,body condition,health,strain type,and genetic mutations.Environmental parameters known to effect production include caging system,bedding,diet,temperature humidity, light cycle, noise and vibration[2,7-9].
As increase in animal exchange occur between institutions,rodent colony managers must take into considerations that new m ice are exposed to unknown microorganisms in the environment that may subclinically or clinically impact their health and reproductive performance. Additional challenges include sharing of incomp lete genetics and breeding history,and the need to establish a colony based on a few individual animals.Finally,creation of novel animal models through genetic manipulation may sometimes render the phenotypic expression and reproductive performance unpredictable. These scenarios can present as hidden problems that interfere with effectivemanagement of a production colony.
When realistic production expectations are not being met,one should take a systemic approach to identify the problems(s).Scrutinize the animals,their environment,and their genetics.Determine if the problem is the result ofmice notmating(e.g.lack of copulatory plug),if themice are not getting pregnant,if pregnancy is noted,but no pups are born,if pups are born but are not raised,or dies before weaning.Or perhaps production level is not meeting the expected number or genotype distribution,model phenotype and performance? If there is a problem perpetuating valuable animals,assisted reproductive technologies (ART)are available for rescue purpose[10].
If the problem is that mice aren’t breeding,examine the breeders within the cage to rule out any age,anatom ical,or health related issues that m ight have interfered with successful mating.One may change one or more environmental parameters(e.g.light cycle, diet type, supplement provision,environmental enrichment,cage/rack location,etc.) to see if there is an improvement.A lternatively,set up the problematic breeders with different partners in new cages.One may introduce young,wild-type animals from vendors into the breeding pool.For older females potentially experiencing prolonged anestrous,it has been suggested that low dose gonadotropin injections may be used in an attempt to“jump start”the estrous cycle.Typically a strain dependent dose that is half the usual dose for prepubescent animals is recommended,but effectiveness is not always guaranteed.Alternatively,ART such as ovarian transplantation,embryo transfer,in vitro fertilization (IVF),and intracytoplasmic sperm injection (ICSI)are commercially available.
If the problem is thatmice are mating as evident by the presence of a copulatory p lug,but does not result in pregnancy,then one should replace the male breeder,or perform IVF after assessing his sperm quality and quantity.Fertilized embryos are then imp lanted into surrogate females to perpetuate a line.If the females do show signs of pregnancy,but do not litter as expected,consider potential causes of abortion or fetal resorption.The possibility of nutritional deficiencies or toxicity,subclinical disease state,genetic manipulation,and environmental stressors should be investigated.
If the problem involves post-natal lethality,check for m ilk-spots in newborn pups to ensure there is no lactation problems with the dam,and that the pups have normal suck ling ability and have been nursing.Diagnostic workup may be necessary to rule out infectious agents of colony health concern,such as rotavirus or mouse hepatitis virus,which are known to cause morbidity and mortality in susceptible preweanlings. If dealing with novel genetically engineered mouse models,it will be important to perform genetic testing on the pups to rule out the possibility of a detrimental phenotype and adjust breeding scheme accordingly.If necessary,consider cross-fostering the pups to salvage valuable litters.Where appropriate,as an alternative to cross-fostering,ovarian transplantation may be used to rescue a line when the colony has been decimated down to unproductive females.Additional tips on crossfostering and ovarian transplantation may be found in the chapter by Pritchett and Taft[2].
In a typical closed inbred colony,the production level will drop as result of inbreeding depression.If all of a sudden a robust production is observed,then one should suspect hybrid vigor and test the colony for potential mismating or genetic contamination.If there is an unexpected distribution of offspring genotypes,for example,lack of homozygous pups from mating parents heterozygous for amutation,it is good practice to retest the parents to confirm their genotype,then consider in utero or post-natalmortality.To confirm suspicion,one could perform timed-mating to check the genotype of embryos or fetus at different gestation stages.
If one is following the production planning calculations,but is still unable to meet production goals,ART may be used to generate a large number of animals within a short period of time.Depending on the availability of the breeders,one could superovulate mice with exogenous hormones,then either collect oocytes for IVF or time-mate the animals to collect stage specific embryos for embryo transfer.For superovulation and IVF protocols, a mouse embryologist should be consulted.Dosage and success of superovulation will be dependent on animal strain,age,and weight.
5 Im portance of Genetic Background and Nom enclature of M ice
One cannot stress enough the importance of genetic background and use of proper nomenclature when working with mouse models.The degree of gene expression,or animal phenotype,can vary depending on the genetic background of the model.Many commonly used inbred strains exhibit strain specific pathologies,or“background lesions,”that can confound the result of genetic manipulation.Further inbred strain characteristicsmay be found under Mouse Genome informatics website hosted by The Jackson Laboratory (www.informatics.jax.org/external/ festing/mouse/STRAINS.shtm l). Finally, the behavior of mice can vary by vendors and production sites,by strains and substrains,and is subject to external influence(i.e.environmental conditions,husbandry practice)under which the animals were raised.Therefore,whenever possible,pay close attention to factors that have the potential to alter the performance of the animalmodel.
The proper mouse nomenclature serves as a unique identifier of mice, convey important technology,line,and producer information.In scientific publications,proper mouse nomenclature should be used in order to ensure reproducibility of that particular study.The use of proper nomenclature is also pertinent for animal purchasing agents to ensure the correct animal models are ordered and used for studies.Knowing the proper nomenclature of themouse model will allow for more effective management of breeding records and pedigree information.A thorough review ofmouse nomenclature may be found in various reference texts[6,11],while the current guidelines are hosted on The Jackson laboratory website(www.informatics.jax.org/nomen).
6 M ain taining Genetic In tegrity
The utilization of genetically defined mice and their proper controls is essential to the validity of an experiment.In any population,the prevalence of various genotype/phenotype is constantly changing,and genetic divergence can occur due to spontaneous mutations,natural selection(though less likely in our controlled laboratory setting),unconscious selection,genetic drift,and population migration.If two populations are separated geographically,their genotype and phenotype will assort independently and likely diverge from one another.Certain phenotype or genotype may become fixed or eliminated from one population or another,and if different populations are assayed over time,one will see that the distribution of genotype/phenotype varied in an unpredictable fashion.
In the management of mouse colonies,sources of genetic variability may result from human error(e.g.genetic contam ination,incomplete inbreeding resulting in residual heterozygosity), intentional genetic manipulation,or the unmonitored accumulation and fixation of spontaneous mutations that occur over time (i.e.genetic drift).This often accounts for the variation seen over time in research results,despite the control of all external parameters.Managers of mouse breeding colonies,especially those working in institutions that maintain small or closed colonies,should be aware of this and implement measures that will control potential sources of genetic variability.
In addition to becoming familiar with specific strain characteristics,training of mouse users should include basic rodent genetics,proper record keeping,and recognition of deviant phenotypes.Biological control measures may include capturing and euthanizing all escaped mice,cryopreservation,and establishment of foundation colonies.Strains of the same coat color should be separately housed whenever possible,or else use color coded cage cards to distinguish between populations.
When appropriate,testing should be performed to monitor the genetic integrity of the colony.Genetic testing can include the zygosity testing for genes(s)of interest(i.e.genotyping)and background testing,which identifies genetic background composition as related to a particular mouse strain.Prior to expansion of a colony using only a few animals received from a collaborator,it is always a good idea to perform initial characterization with regards to the genotype and/or genetic background of the mice to make sure the animals are what you expect,as well we determine which substrain they are in case it is necessary to acquire wild-type mice from a vendor.Discussions related to genetic monitoring,including various techniques used for genetic testing in mice,may be found in many published references[12-15].
7 M eeting Production Expectations
In order to meet production expectations,it is important to understand the purpose of the breeding colony.For example,whether the purpose is to supply animals involved in embryo transfer projects,to create and expand a specific founder/chimera line,to generate cohorts of animals for scientific experiments,or to simply maintain backup colonies when large scale production is occurring elsewhere.The goals for any breeding colony also needs to be clearly defined,whether there is a specific number of animals required over a given period of time,and what the required characteristics are(e.g.sex,age rage,genotype).Furthermore,in order to set realistic expectations for any production colony,information regarding the background strain,its production indices,reproductive lifespan,maternal attributes,availability of breeders,mating systems and breed schemes,model phenotype and health statusmust all be taken into consideration.
Historic information regarding the reproductive characteristics of different basic mouse strains may be found in texts by L.Silver and W.White[3,5].Tables 1 and 2 are adapted from published texts for the purpose of demonstrating the process for production planning calculations.Data relevant to one's own mouse colony should ideally be derived from breeding record data maintained within your own institution,since data will vary due to the combined interaction of animal reproductive ability,production systems,and the breeding environment.
8 Production Planning
To plan production,one needs to know the number and the characteristics of animals needed,as well as the productive mating frequency for the strain.For examp le,if breeding BALB/cJ m ice,or genetically manipulated mice on BALB/cJ background,where the anticipated productive mating frequency is 47%(table 1),onemay expect that after setting 10 females up to breed,only 4 to 5 mice will produce litters.Therefore,if a study requires a m inimum of 10 pregnant females,itmay be necessary to set up twice asmany animals to breed.Then,based on the actual production outcome,the breeding population may be adjusted accordingly.
Production index(PI),on the other hand,refers to the number of animals weaned per female in the breeding colony per week(i.e.PI=pups weaned/ female/week).Mice of C57BL/6 background has a PI of 0.5(table 2).Therefore,in a scenario requiring continuous production,one would expect to wean 0.5 pups each week per breeder female.To calculate the PI for an internal colony,simply count the number of weaned animals,and not just those born,over a number of weeks,and all of the females used in production,not just those with litters.The longer span of breeding data collected,the more accurate the calculated number will reflect the colony status.
During production p lanning exercises, the mathematical equation used can be listed as follows:# females x PI= #pups weaned/week.If productive mating frequency information is available,it should be taken into consideration in the arithmetic in anticipation that some mice set up to breed may be unable to mate,and are considered infertile[6].
As an examp le,if aresearcher needs 20 homozygous(HO)mutantmales on a C3H background every week for 6 weeks,and only heterozygous(HE) mice are available for breeding,what is the m inimum size of the breeding colony required?One should understand that only 25%of the animals produced from a HE x HE breeding scheme will be of the desired genotype.Assuming equal distribution of males to females in a litter,only 12.5%(or 1/8)of the offspring produced will be useful.Therefore,each week 20 x 8=160 pups will need to be produced in order to meet production goal.Using the equation# females x PI=#pups weaned/week,where#pups is 160 and PI is 0.8 for C3H mice,then#females= 160/0.8=200.In addition,knowing that productive mating frequency is 86%for C3H/J substrain(table 1),it may be necessary to increase the number breeder females to 233(e.g.200 divided by 0.86)in anticipation that roughly 14%of the animals set up may not breed as expected.This simple arithmetic provides a rough estimate for the number of breeding females required to sustain the production goal of 20 homozygote males each week for 6 weeks.
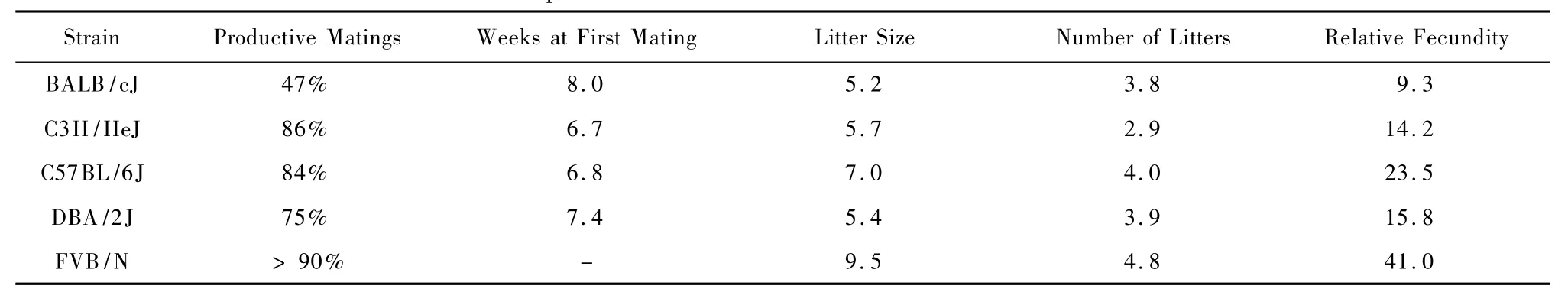
Tab.1 Reproductive characteristics of common inbred strains[5]
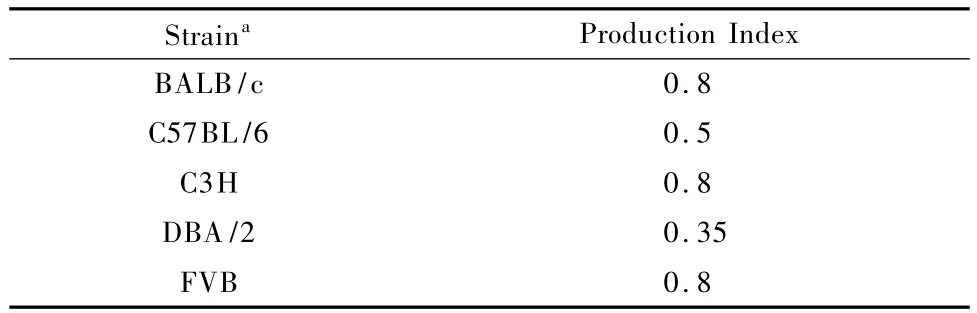
Tab.2 Typical production indices for common inbred strains[3]
Ultimately,there is no guarantee that the animals will breed when expected,produce the number of offspring expected,or produce animals of the expected genotype or sex.Therefore,more animalsmay need to be included,especially if one is setting aside animals as future breeders in addition to producing study animals.For more information and examples of production p lanning,please refer to the book chapter by W.White,[3]appendix B in the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research,[6]or the resourcemanual available from The Jackson Laboratory[16].
9 Disaster Planning
When managing rodent breeding colonies,especially valuable mouse lines,it is important to plan against disasters,including natural disasters that can cause one to lose an entire colony,or potential genetic ormicrobiological contamination within a colony.One practice is to split colonies and manage animals in different rooms or different facilities.Another practice is to cryopreserve the embryos or sperm of valuable lines.The choice to cryopreserve embryos vs.sperm will be dependent on one's ability to reconstitute the line in the future,the genotype requirements postreconstitution,and the availability of donor animals.Compared to splitting colonies,cryopreservation has additional advantages.For example,it is a viable,cost-effective long-term storage solution that prevents the loss of a line,while allowing other active colonies to occupy vivarium space.This practice not only slows down genetic drift within a colony,but also rederives the colony when reconstituted,sharing the health profile of the recipient animals.Finally,frozen germplasms may be shipped to collaborators with less cost and more ease as compared to shipping live animals.
Acknow ledgem en t
The author wish to thank Dr.Cecilia Hui Wang for her translation assistance.
[1]Murray KA,Parker NJ.Breeding genetically modified rodents: tips for tracking and troubleshooting reproductive performance[J].Lab Animal,2005,34(4):36-41.
[2]Pritchett KR,Taft R.Reproductive Biology of the Laboratory Mouse[M].In:Fox JG,Barthold SW,Davisson MT,et al.The Mouse in Biomedical Research,Volume 3:Normative Biology,Husbandry,and Models(2ndedition).Burlington,MA: Academic Press,2007.
[3]White WJ.Management and Design:Breeding Facilitie[M].In:Fox JG,Barthold SW,Davisson MT,et al.The Mouse in Biomedical Research, Volume 3: Normative Biology,Husbandry,and Models(2ndedition).Burlington,MA: Academic Press,2007.
[4]Festing MFW,Peters AG.Animal Production and Breeding Methods[M].In:Poole T.The UFAW Handbook on the Care and Management of Laboratory Animal,Volume 1:Terrestrial Vertebrates(7thedition).Oxford,UK:Blackwell Science Ltd.,1999.
[5]Silver LM.Mouse Genetics[M/OL].New York,NY:Oxford University Press,1995.http://www.informatics.jax.org/silver/
[6]National Research Council.Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research[M].Washington,D.C:The National Academies Press,2003.
[7]Baumans V.The Laboratory Mouse[M].In:Poole T.The UFAW Handbook on the Care and Management of Laboratory Animal,Volume 1: Terrestrial Vertebrates(7thedition).Oxford,UK:Blackwell Science Ltd.,1999.
[8]Lipman NS,Perkins SE.Factors That May Influence Animal Research[M].In:Fox JG,Anderson LC,Loew FM,et al.Laboratory Animal Medicine(2ndedition).Burlington,MA: Academic Press,2002.
[9]Berry ML,Linder CC.Breeding Systems: Considerations,Genetic Fundamentals,Genetic Background,and Strain Types[M].In:Fox JG,Barthold SW,Davisson MT,et al.The Mouse in Biomedical Research,Volume 1:History,W ild Mice,and Genetics (2ndedition).Burlington,MA: Academic Press,2007.
[10]Lloyd KCK.Gamete and Embryo Manipulation[M].In:Fox JG,Barthold SW,Davisson MT,et al.The Mouse in Biomedical Research,Volume 1: History,W ild Mice,and Genetics(2ndedition).Burlington,MA:Academic Press,2007.
[11]Eppig JT.Mouse Strain and Genetic Nomenclature: an Abbreviated Guide[M].In:Fox JG,Barthold SW,Davisson MT,et al.The Mouse in Biomedical Research,Volume 1: History,W ild Mice,and Genetics(2ndedition).Burlington,MA:Academic Press,2007.
[12]Festing MFW.Introduction to Laboratory Animal Genetics[M].In:Poole T.The UFAW Handbook on the Care and Management of Laboratory Animal,Volume 1:Terrestrial Vertebrates(7thedition).Oxford,UK:Blackwell Science Ltd.,1999.
[13]Sharp JJ,Sargent EE,Schweitzer PA.Genetic Monitoring[M].In:Fox JG,Anderson LC,Loew FM,et al.Laboratory Animal Medicine (2ndedition). Burlington,MA: Academic Press,2002.
[14]Sundberg JP,Ichiki T.Genetically Engineered Mice Handbook[M].Boca Raton,FL:CRC Press,2006.
[15]Fox RR,W iles MV,Petkov PM.Genetic Monitoring[M].In: Fox JG,Barthold SW,Davisson MT,et al.The Mouse in Biomedical Research,Volume 1:History,W ild M ice,and Genetics (2ndedition).Burlington,MA: Academic Press,2007.
[16]The Jackson Laboratory.Breeding Strategies for Maintaining Colonies of Laboratory Mice,A Jackson Laboratory Resource Manual[M].Bar Harbor,ME:The Jackson Laboratory,2007.

