软珊瑚Nephtheasp.中新生物碱分离与结构鉴定
王长军,徐石海,廖小建,林 慧
暨南大学化学系,广州 510632
Introduction
The soft corals of the genusNephtheabelongs to coelenterate phylum,anthozoan,soft coral suborder,nephtheidae family.Besides the cembrene terpenoid[1],othermetabolis m have been continuously obtained,such as steroids,quinone,terpene and ceramides[2-11].In our previous research for bioactive metabolites from marine invertebrates,EtOAc extract of Chinese soft corals, Nephtheasp.,showed cytotoxic activity.Two alkaloids with cytotoxic toward a limited panel of cancer cell lineswere isoilated and identified.Among Octocorallia the genusN ephtheaof the family comprises of a large variety of species.The current literature is far from enough identification ofNephtheamaterial into the species level;a study based on a diverse collection from numerous Islands is still ongoing.Alkaloids have never been isolated from the genusNephtheabefore on the base on the searching result from SciFinder Scholar searches.The project aimed at isolating antitumour compound from the Xisha soft coralNephtheasp.led to the discovery of a new bis-piperidone framework alkaloid,Nephoxaloid(1),and the known alkaloid Caulerpin (2).The caulerpin were found in several algae[12-13],but it has been obtained in coral for the first t ime.Itmay be the product of intergrowth of coral and algae.In thiswork we described the isolation and structure elucidation of two alkaloids and the cytotoxic activity of them.
Exper imental
Sample and General
The soft coralN ephtheasp.was collected by SCUBA diving off a reef at a depth of 15-20 m at Xisha Island, South China Sea,PR China,in May 2001.The texture is soft,leathery,and very flexible.The species was identified by professor Ren-Lin Zhou (Academe of South Ocean of Science and Technology in China).
The IR spectra were recorded on a Perkin-ElmerNicol FT-50X spectrometer.The melting points are uncorrected.1H and13C NMR spectrawere recorded on aBruker Avance DRX 500 NMR spectrometer using T MS as internal standard.MS spectrawere obtained on aVGAtospec spectrometer.Column chromatography was carried out on Merck Silica gel(200-400 mesh),and the HF254Silica gel for TLC was provided by Sigma Co. Ltd.Sephadex LH-20 (18-110μm)were obtained from Phar macia.
Isolation and Purification
The soft coral(6 kg,wet weight)was extracted with EtOH(total amount 10 L)in a blender.The combined extracts,after filtration,were concentratedin vacuum until dried to give a black residue(120 g).The crude residue was partitioned be tween water and ethyl acetate.The organic layer was concentrated under vacuum to yield greenish gum (35 g),and this residue was then subjected to silica gel column chromatography using petrol ether(60-90℃)containing increasing amount of EtOAc and CHCl3containing increasing amount ofMeOH as an eluent,respectively.The fraction eluted with MeOH was subjected to Sephadax LH-20 (MeOH/H2O 7:3),and followed byODS open column (MeOH/H2O 6:4)to yield(1),black green flack solid(4 mg),which showed one spot in TLC(CHCl3/ MeOH 8:2,Rf 0.34).The fraction eluted with petroleum-EtOAc(3:7)yield to Sephadax LH-20(MeOH/ H2O 7:3),and followed byODS open column(MeOH/ H2O 6:4)to yield(2),orange red pris ms(15 mg).
Identification of Structure
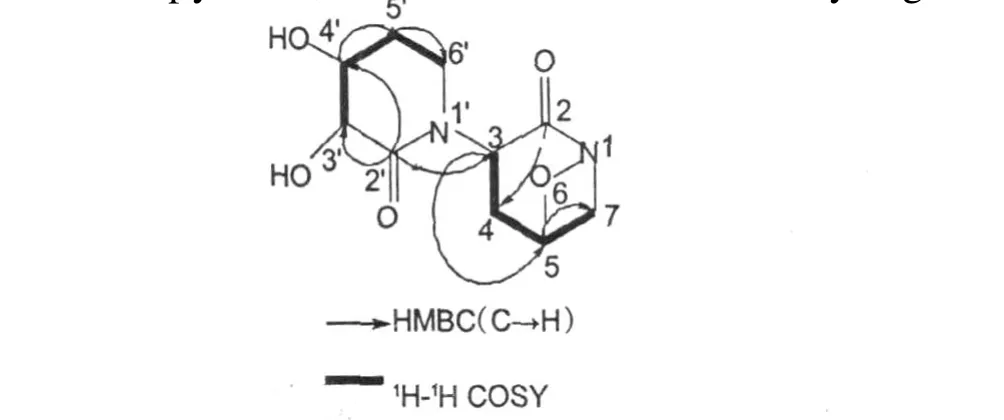
Fig.1 Key1H-1H COSY and HM BC correlations for compound 1
Nephoxaloid(1)was obtained as black green flack solid,mp.198-200℃.Its HRE IMS exhibited a molecular ion peak atm/z243.0976[M +H]+,corresponding to the molecular for mula for C10H15O5N2+ (calcd.243.0981),indicating 5 degrees of unsaturation.Typical IR absorptions at 3439,1665 cm-1demonstrated the presence of hydroxyl and carbonyl groups, respectively.Comprehensive analysis of13C NMR and DEPT data suggested 10 carbon signals,which were classified into two quaternary carbonyl carbons(δC169.1,167.7),three methine carbons bearing oxygen (δC72.5,69.6,67.2),one methine carbon(δC60. 0),and fourmethylene carbons(δC54.7,43.6,37.6, 32.9).Besides two degrees of unsaturation were ascribed to two carbonyl carbons,3 degrees of unsaturation could only be assigned to the presence of 3 rings, for there is no alkene and alkyne carbon.The skeleton of(1)was further determined by analysis of1H-1H COSY and HMBC spectra.The1H-1H COSY and HSQC spectra indicated connectivity of two segments-CHCH2CHCH2-(A)and-CHCHCH2CH2-(B)drawn with bold bonds in Figure 1.Based on the signal atδC54.7 and the cross peak of C-2/H-4b,and the cross peak of C-3/H-4a,appeared in HMBC spectrum,sixmembered ring could be formed by segment A and an imide group.Based on the signal atδC43.6,the chemical shift of which matched well with the methylene lying adjacent to the imide,and the cross peak of C-2′/ H-3′,H-4′,H-6′,appeared in HMBC spectrum,another six-membered ring could be deduced by segmentB togetherwith another imide group(Table 1,Figure 1). Based on the signal atδC69.6,a methine carbon bearing oxygen,and the molecular for mula account for only two hydroxyls,it was determined that the oxygen mentioned must be attached to amide group for ming a four member ring.Because there was no hydrogen of amide and with the cross peak of C-2′/H-3 appeared in HMBC spectrum,two six-membered rings should be linked between C-3 and N-1′.Therefore,the compound 1 was assigned as 3-(3,4-dihydroxy-2-oxopi-peridin-1-yl)-6-oxa-1-aza-bicyclo[3.1.1]heptan-2-one,which was also named Nephoxaloid.In additional,the mass spectrum of 1 contained ion peaks atm/z242,226,198, 156,154,112 and 55 also has confirmed its structure. Compound(2)(Figure 2)obtained as orange red pris ms,mp.316-318℃,was identified as caulerpin by comparison of its spectral data with literature values[12,13].The NH proton resonates was at presence ofδ 11.57 in pyridine,because of the for mation of hydrogen bonds at inner molecule.The13C NMR data including multiplicities by DEPT of compound 2 is also near equalwith those published.Caulerpin,a dimerof indole-3-acrylic acid,behaves much like the indole auxins. Bioassy showed that caulerpin is an effective growth regulator[13].
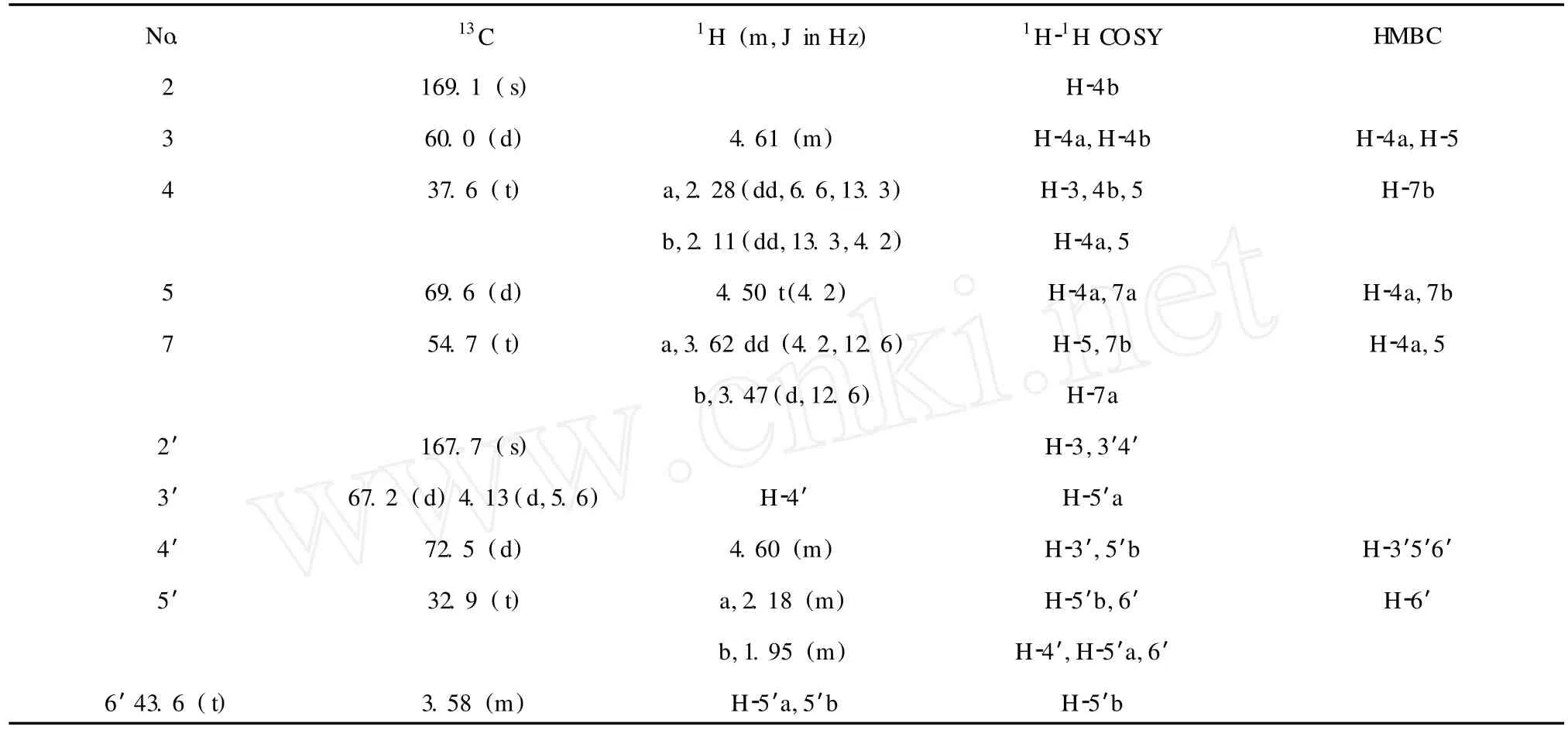
Table 1 NM R data forNephoxaloid(1)in CD3OD
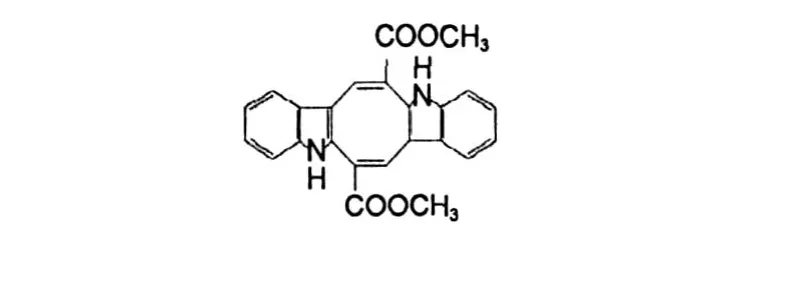
Fig.2 Chem ical structure of compound 2
Besides its growth regulator reported in published,another interesting results of the cytotoxicity of caulerpin (2)against the MCG,MCF,LOVO,and Bel-7402 cell lines have been found,it showed cytotoxicity against the four cancer cell lines(IC5012.57,29.10,12.46 and 6.11μg/mL,respectively).
Results and D iscussion
Nephoxaloid 1,possessing an unprecedented tricycleskeleton,together with the known alkaloid caulerpin (2)have been isolated from the soft coralNephthea sp.collected from the Xisha island of China.Their structures were established by NMR and MS spectroscopic analysis.The cytotoxic activity of 1 against the Bel-7402,LOVO andMCF-7 cell lineswas studied and it exhibited moderate cytotoxicity against the growth of this cancer cell lines(IC5014.3,28.8 and 30.5μg/ mL,respectively).Besides its growth regulator reported in published,another interesting results of the cytotoxicity of 2 against theMCG,MCF,LOVO,and Bel-7402 cell lines have been found,it showed cytotoxicity against the four cancer cell lines(IC5012.57,29.10, 12.46 and 6.11μg/mL,respectively).
1 David JV,Neal R.Cembrene-A and cembrene-C from a soft coral,Nephtheaspecies.J O rg Chem,1978,43:1614-1616.
2 Kiyagawa I,Cui Z,SonBW.MarineNatural Products.XVⅡ. Nephtheoxydiol,a new cytotoxic hydroperoxygermacrane sesquiterpene,and related sesquiterpenoids from an Okinawan soft coralofNephtheasp.(Nephtheidae).Chem Phar m Bull, 1987,35:124-135.
3 Gross H,Kehraus S,NettM.New cytotoxic cembrane based diterpenes from the soft coralsSarcophyton cherbonnieriand Nephtheasp..O rg B iom ol Chem,2003,1:944-949.
4 RayAK,Datta PK,Das T.Isolation ofmeso-1,3-diphenyl-1, 3-propanediol from a soft coralNephtheasp..J Nat Prod, 1991,54:854-855.
5 Patra A,MajumdarA.Secondarymetabolites of a soft coral( Nephtheasp.)of the bay of Bengal.RKIVOC,2003,9:133-139.
6 Koren-Goldshlager G,Klein P.Sindurol and nephthoside:new tetraprenyltoluquinols from the soft coralSinularia duraand Nephthea sp..J Nat Prod,1996,59:262-266.
7 Duh CY,Wang SK,ChuMJ,et al.Cytotoxic sterols from the soft coralNephtheaerecta.J Nat Prod,1998,61:1022-1024.
8 El-GamalAH,Wang SK.New Nardosinanes and 19-oxygenated ergosterols from the soft coralNephtheaarmata collected in Taiwan.J Nat Prod,2004,67:1455-1458.
9 Kapojos MM,Mangindaan REP,Nakazawa T,et al.Three new nardosinane type sesquiterpenes from an Indonesian soft coralNephtheasp..Chem PharBull,2008,56:332-334.
10 Cheng SY,Huang YC,Wen ZH,et al.New 19-oxygenated and 4-methylated steroids from the Formosan soft coral Nephthea chabroli.Steroids,2009,74:543-547.
11 Cheng SY,Huang YC,Wen ZH,et al.Novel sesquiterpenes and norergosterol from the soft coralsNephtheaerecta and Nephthea chabroli.Tetrahedron Lett,2009,50:802-806.
12 GovenkarMB.Constituents of chondria armata.Phytochem istry,2000,54:979-981.
13 Xu XH(徐效华),Su JY(苏镜娱).The separation,identification and bioassay of caulerpin.Zhongshan Daxue Xuebao, Ziran Kexueban(中山大学学报,自科版),1996,35:64-66.
