Pharmacokinetic Study of a Novel Recombinant Human Granulocyte Colony-stimulating Factor in Rats△
Xiao-xiao Liu and Yong-ping Jiang,2*
1Biopharmaceutical R&D Center,Chinese Academy of Medical Sciences &Peking Union Medical College,Suzhou 215126,China
2Biopharmagen Corp.,Suzhou 215126,China
HUMAN granulocyte colony-stimulating factor(G-CSF) is an endogenous glycoprotein which regulates survival,proliferation,and differentiation of neutrophilic granulocyte precursors,functionally activates mature neutrophils,and maintains normal counts of neutrophils in blood.1,2Human G-CSF had been successfully expressed in and purified from CHO cells andE.coli,and was approved for clinical use in 1991.3Human G-CSF is used as a therapeutic drug for cancer patients who receive myelosuppressive chemotherapy and those with congenital neutropenia.4,5Also,the drug is currently under investigation for treatment of cerebral ischemia and amyotrophic lateral sclerosis.6,7
Due to its intrinsic instability,8,9G-CSF should be excessively and/or frequently administered to patients in order to maintain a plasma concentration which is high enough to achieve therapeutic effects.This administration regimen not only causes inconvenience and pain in patients,but also increases the chance for infections.A mutant derivative of granulocyte colony-stimulating factor,rhG-CSFa,which exhibits greater biological activity and enhanced stability in myelo-suppressed monkeys,was obtained previously through additional site-directed mutagenesis.10In this study,the pharmacokinetics of rhG-CSFa was investigated after intravenous or subcutaneous administration of it at three different doses in rats.
MATERIALS AND METHODS
Animals
All animals used in this study were obtained from the Experimental Animal Center of Soochow University (Suzhou,China).Female Balb/c mice (specific pathogen-free,SPF) aged 8-10 weeks old,male and female Sprague-Dawley rats (clean animal,CL) weighing 180- 220 g,and female New Zealand big ear rabbits (CL) weighing approximately 2 kg were used.
Reagents
Fetal calf serum and RPMI 1640 medium were purchased from Thermo Fisher Scientific Corp (Beijing,China).Polyethylene glycol (PEG),aminopterin (50×),hypoxanthine,thymine,streptavidin-peroxidase,and o-phenylendiamin were purchased from Sigma (St.Louis,USA).Biotin goat anti-mouse IgG and alkaline phosphatase (AKP) goat anti-mouse IgG were purchased from Biolegend (San Diego,CA,USA).Horseradish peroxidase (HRP) goat anti-rabbit IgG and bovine serum albumin (BSA) were purchased from Dingguo biotechnical corp.(Beijing,China).Penicillin-Streptomycin was purchased from Gibco (NY,USA).NBT/BCIP was purchased from Bio-RAD (Richmond,CA,USA).Cell culture flasks,96-well plates,and 24-well plates were purchased from Corning (NY,USA).
Cell lines and cell culture
Sp2/0 myeloma cells were provided by the Institute of Genetics,Fudan University (Shanghai,China).They were cultured in RPMI 1640 medium containing 10% fetal calf serum.
Preparation of rabbit anti-sera specific for rhG-CSFa
Female New Zealand big ear rabbits received five subcutaneous injections (initial dose of 0.25 mg and subsequent dose of 0.5 mg) of rhG-CSFa at 2-week intervals.The rhG-CSFa solution was emulsified with Freund’s complete adjuvant for the initial injection,and then with Freund’s incomplete adjuvant for the subsequent injections.Blood was collected 8 days after the last subcutaneous injection and the rhG-CSFa specific anti-sera were prepared and stored at-20°C.When used in enzyme-linked immunosorbent assay (ELISA),the anti-sera were diluted at 1∶1000 (anti-sera∶PBS).
Preparation of monoclonal antibodies (MAbs) specific for rhG-CSFa and Western blotting analysis
Female Balb/c mice were immunized with rhG-CSFa,and their spleen cells were obtained and fused with sp2/0 myeloma.The cells were then cultured in HAT medium for hybridoma selection,and the supernatants of the cultures were tested by ELISA for specific positive hybridomas.The positive cells were cloned by the limiting-dilution method to obtain the MAb cell lines.The specific activity of the antibody was confirmed by Western blotting analysis.11
Detection of rhG-CSFa concentrations in rat serum by ELISA
Rabbit anti-rhG-CSFa serum was added to each well of a 96-well plate,and incubated overnight at 4°C.A BSA solution (0.2%) was added for blocking.Then 100 μL of serum sample or rhG-CSFa standard diluted in phosphate-diluted saline (PBS) was added to the wells,and incubated for 1 hour at 37°C.After washing each well with 200 μL of buffer for 3 times,100 μL of MAb culture supernatant was added to the wells,followed by the avidin-biotin system ELISA.11
Determination of standard curve of rhG-CSFa and wild type (WT) G-CSF by ELISA
rhG-CSFa or WT G-CSF standards at 9 different concentrations were prepared by serial dilution with PBS;the concentrations were as follows:0,19.5 ng/mL,39.1 ng/mL,78.1 ng/mL,156 ng/mL,313 ng/mL,625 ng/mL,1 250 ng/mL,and 2 500 ng/mL.Absorbances were measured at 490 nm with a Bio-Rad 3000 reader.The standard curves were constructed by plotting the absorbance values against the standard concentrations of rhG-CSFa or WT G-CSF.
Analysis of pharmacokinetic parameters
Pharmacokinetic parameters were determined by fitting the serum drug concentration-time data to a one-or two-compartment model,with use of the Winnonlin 5.2 software.Accumulated amount of the drug in rat circulating blood in a given time was measured by area under concentration-time curve (AUC).Clearance and elimination half life (T1/2(β)) were used to describe the rate of elimination of drugin vivo.Volume of distribution of central compartment (Vc) showed how well the drug distributedin vivo.Distribution half life (T1/2(α)) was the time it took for the durg to distribute into the whole body.Within the safety range,the value of maximum concentration (Cmax) would correlate well with the therapeutic effects of a drug.Bioavailability (F),which was determined by the AUC values,and which shows how well a drug is absorbed,is also an important parameter used to measure a drug’s bioactivity and its therapeutic effect.
Stability of rhG-CSFa and WT G-CSF in rat whole blood or serum in vitro
In proteolytic assay using rat whole blood,rhG-CSFa or WT G-CSF was diluted in rat whole blood containing 3.8%sodium citrate as anticoagulant,with a final concentration of 1 250 ng/mL.The samples were incubated at 37°C,and 50 μL of the samples were collected into eppendorf tubes at 0,3,6,9,12,15,18,21,24,27,and 30 minutes.The tubes were stored at 4°C to stop the proteolytic reactions while the remaining samples were collected,all of the samples were then centrifuged at 18 000×gfor 5 minutes at 4°C,and the supernatants were used for ELISA.
To determine proteolytic rates of rhG-CSFa and WT G-CSF in rat serum,rhG-CSFa or WT G-CSF was added to the serum at a final concentration of 1 250 ng/mL,and the assay was performed as described above for the whole blood.
Statistical analysis
Pharmacokinetics data from rhG-CSFa or WT G-CSF were compared by one-way analysis of variance,andttest were performed for the comparison between the two means of the same parameters of rhG-CSFa and WT G-CSF for all the three doses.The null hypothesis was rejected when thePvale was less than 0.05.
RESULTS
Production and specificity of MAb to rhG-CSFa
Ten positive hybridoma cell lines were selected by ELISA,and three of them with higher optical density (OD) readings were further monocloned with the limiting-dilution method.Culture supernatant from the MAb cell lines was analyzed for specificity on Western blots;rhG-CSFa,endostation (negative control),and inclusion body of WT G-CSF were electroblotted onto nitrocellulose filters.Results showed that specific bands with molecular weight less than 20 kD were detected in samples of rhG-CSFa and WT G-CSF,and no band was observed in sample of endostatin (Fig.1).
Standard curve of rhG-CSFa and WT G-CSF detection by ELISA and the lowest detectable concentration
Standard curves of rhG-CSFa and WT G-CSF are shown in Figure 2,and the lowest detectable concentration of this ELISA method was 0.125 ng/mL.
Rates of recovery and precision
When rhG-CSFa was added to rat serum,the recovery rate of added rhG-CSFa ranged from 95% to 104%.Coefficients of within-assay and between-assay variations were 2.4%-7.4% and 9.4%-15.8%,respectively.

Figure 1.Western blot analysis of 5H6-B7 monoclonal antibody.
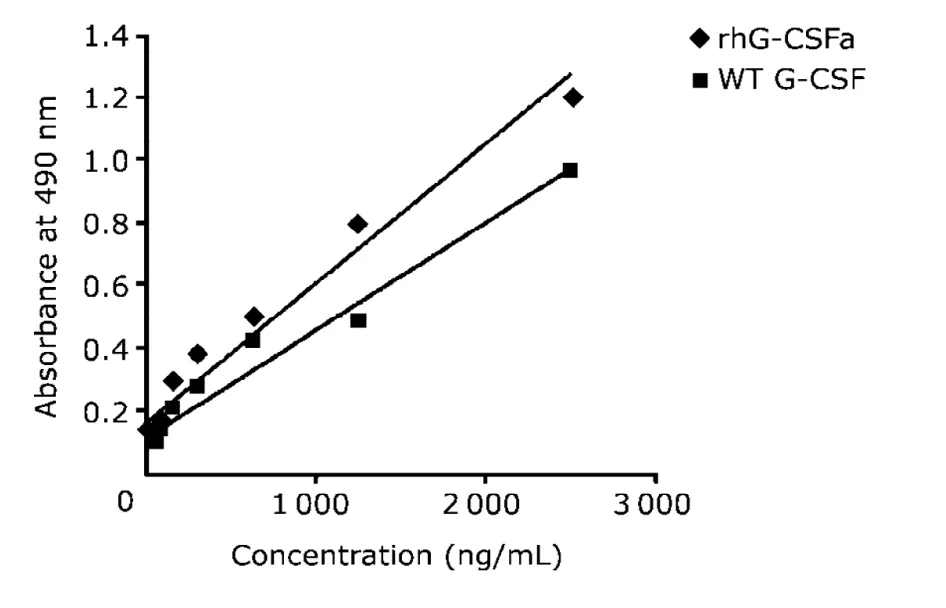
Figure 2.Standard curves of rhG-CSFa and WT G-CSF detection by ELISA.
Pharmacokinetics of rhG-CSFa and WT G-CSF in rats following intravenous or subcutaneous administration
Intravenous administration
After intravenous administration of rhG-CSFa or WT G-CSF at three different doses (20,50,100 μg/kg),the pharmacokinetics of rhG-CSFa and WT G-CSF in rats were studied.Serum concentration-time profiles of rhG-CSFa and WT G-CSF showed that as soon as the drugs were distributed into blood,their concentrations reached the peak value,and then reduced slowly (Fig.3).Corresponding pharmacokinetic parameters are presented in Table 1.Analysis of variance showed that the means of AUC,T1/2(α),and T1/2(β)of rhG-CSFa at the three doses were all significantly different from those of WT G-CSF (P<0.05).
Subcutaneous administration
After subcutaneous administration of rhG-CSFa or WT G-CSF at three different doses (20,50,100 μg/kg),the pharmacokinetics of rhG-CSFa and WT G-CSF in rats were studied.Concentration-time curves of rhG-CSFa and WT G-CSF are shown in Figure 4.Different with intravenous administration,subcutaneous administration took a period of time (about 1.5-2 hours) for the concentration of rhG-CSFa or WT G-CSF in plasma to reach peak values.Corresponding pharmacokinetic parameters are presented in Table 2.Analysis of variance showed that the means of F,Cmax,and T1/2of rhG-CSFa at the three doses were all significantly different from those of WT G-CSF (P<0.05).
Stability studies for rhG-CSFa and WT G-CSF in rat whole blood and serum in vitro
Stability studies were performed by measuring proteolytic rates of rhG-CSFa and WT G-CSF in rat whole blood and serum.Resultsshowed that the rates for rhG-CSFa and WT G-CSF degradation in serum were 13.398 and 19.548 ng·mL-1·min-1,repectively;while in whole blood,the values were 10.212 and 18.292 ng·mL-1·min-1,respectively(Fig.5).
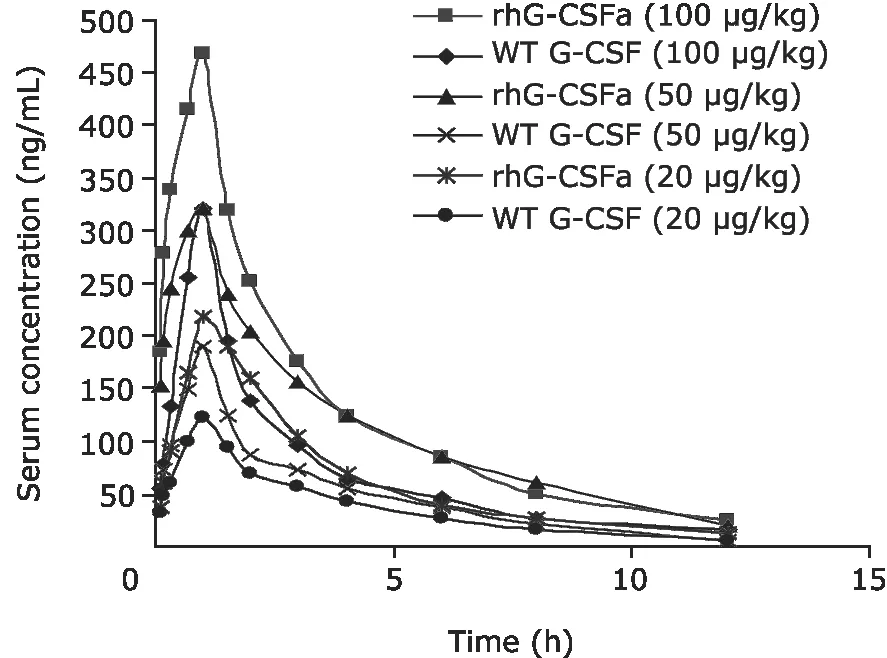
Figure 4.Serum concentration of rhG-CSFa and WT G-CSF after subcutaneous administration in rats.
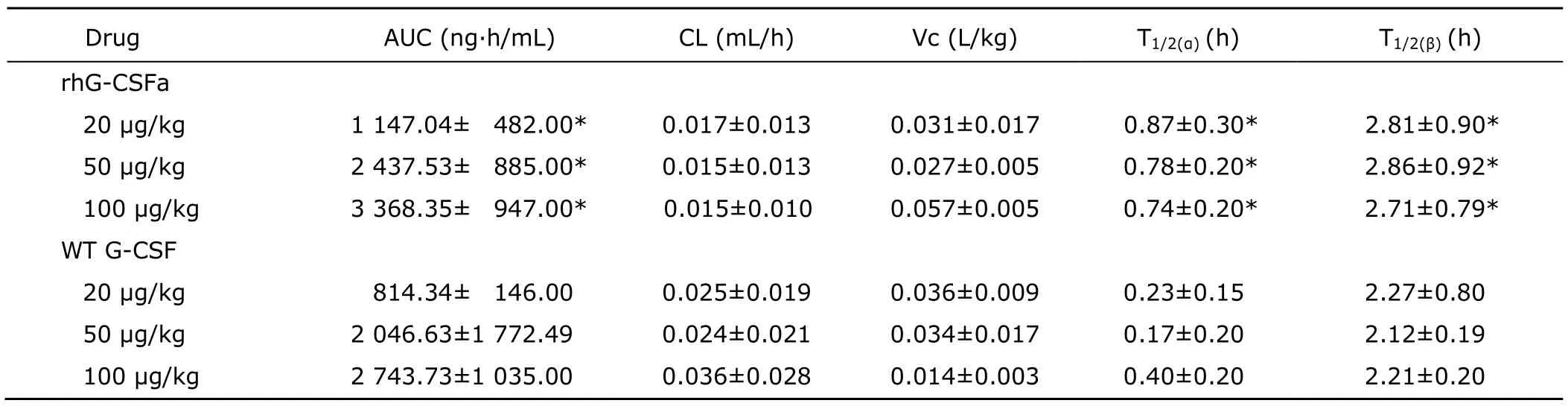
Table 1.Pharmacokinetic parameters of rhG-CSFa and WT G-CSF after intravenous administration in rats§
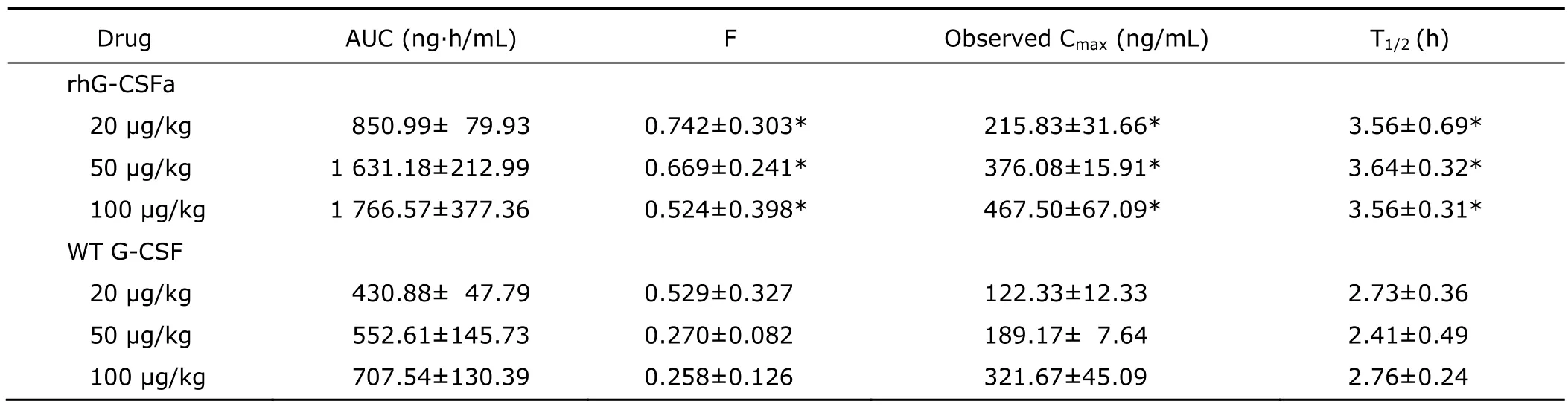
Table 2.Pharmacokinetic parameters of rhG-CSFa and WT G-CSF after subcutaneous administration in rats§
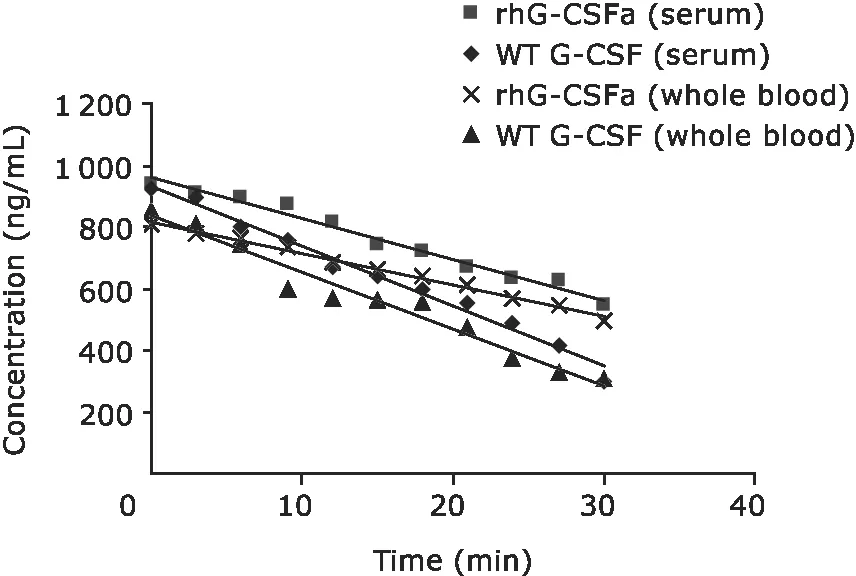
Figure 5.Proteolytic rates of rhG-CSFa and WT G-CSF in whole blood and serum in vitro.
DISCUSSION
In this study,a sandwich ELISA method was used for detecting rhG-CSFa or WT G-CSF.This method was found to be up to 30 times more sensitive than the bioassay method previously reported.12The pharmacokinetics of rhG-CSFa after intravenous or subcutaneous administration to SD rats were studied at doses of 20,50,and 100 μg/kg.For comparison,the WT G-CSF was studies in parallel.Results showed that for the intravenous route,the concentrations of rhG-CSFa or WT G-CSF in the plasma reached their peak values as soon as the drugs were distributed into blood,and the drugs were then slowly eliminated.In contrast,after the subcutaneous administration,the drugs were slowly absorbed from the injection site into blood,and the concentrations in rat plasma reached peak values at 1.5-2 hours after injection.No matter which administration route was selected,the maximum concentrations of rhG-CSFa were significantly higher than those of WT G-CSF.This increase in Cmax may be mainly due to the different elimination rates of the two drugs in rats.One of the clearance mechanisms of rhG-CSFin vivowas receptor-mediated endocytosis and degradation by immature and mature neutrophils.This mechanism is expected to show high-affinity but low-capacity,and be saturated at doses≥10 μg/kg.8Another mechanism may be nonspecific proteolytic degradation or excretion,which would be a high-capacity elimination mechanism and would not be saturated at a dose of 100 μg/kg.Since the administered doses of rhG-CSFa and WT G-CSF in this study were all higher than 10 μg/kg,the elimination mechanismin vivofor these two drugs may mainly involve the role of a series of proteases.
There are many types of proteases in blood,which could degrade protein drugs such as G-CSF.In vitroproteolytic assays showed that the degradation rate of rhG-CSFa in either serum or whole blood was slower than that of WT G-CSF,which indicates that rhG-CSFa has an enhanced stability compared with WT G-CSF.In whole blood,both rhG-CSFa and WT G-CSF were more slowly eliminated than in serum,probably due to the binding of rhG-CSFa or WT G-CSF to the target cells in blood.Thus,the significantly slower rate of rhG-CSFa degradation compared to that of WT G-CSF may also indicate that the binding ability of rhG-CSFa to its receptor is stronger than that of WT G-CSF.Overall,the results from bothin vitroandin vivostudies suggested that rhG-CSFa had a slower clearance rate,a higher plasma concentration,and a longer half life.
Since rhG-CSFa has enhanced stability in plasma,it has a better bioavailability than that of WT G-CSF.On the other hand,bioavailability of either rhG-CSFa or WT G-CSF actually decreased with the increase of drug doses.This result seems to be consistent with the previous report that,when counts of neutrophils in blood became higher than normal level,elimination of G-CSF would become faster,possibly resulting from a feed-back regulatory mechanism.13
The pharmacokinetic characteristics of rhG-CSFa support the design idea of this novel derivation.As a result of additional and site-directed mutagenesis,rhG-CSFa contained four additional amino acids,which may enhance the binding ability of rhG-CSFa to its target receptors,and/or decrease its proteolytic degradation.In addition,native G-CSF contains five cysteine residues,with one cysteine residue (Cys 17) having a free sulfhydryl group.The presence of this cysteine residue may increase the frequency of mismatch between intra-molecular disulfide bonds,or formation of oligomers through an inter-molecular disulfide bond,leading to the loss of the activity of recombinant G-CSF.In rhG-CSFa,the cysteine 17 was substituted with alanine,thus eliminating the aforementioned problems with disulfide bond formation.We believe that these two structural characteristics explain why rhG-CSFa had better therapeutic effects in pre-clinical studies.10
In this study,we have confirmed that rhG-CSFa has a longer half life and better bioavailability than WT G-CSF.These findings provide the foundation for future mechanistic studies aimed at the attainment of a better understanding of the factors that control the half life and decreased proteolytic degradation of rhG-CSFa.
ACKNOWLEDGEMENT
We thank professors Xin-xin Ding and Qing-yu Zhang of the State University of New York at Albany for their helpful discussions and suggestions for this study.
1.Dong F,van Buitenen C,Pouwels K,et al.Distinct cytoplasmic regions of the human granulocyte colony-stimul-ating factor receptor involved in induction of proliferation and maturation.Mol Cell Biol 1993;13:7774-81.
2.Kaushansky K.Lineage-specific hematopoietic growth factors.N Engl J Med 2006;354:2034-45.
3.Welte K,Gabrilove J,Bronchud MH,et al.Filgrastim(r-metHuG-CSF):the first 10 years.Blood 1996;88:1907-29.
4.Smith TJ,Khatcheressian J,Lyman GH,et al.2006 update of recommendations for the use of white blood cell growth factors:an evidence-based clinical practice guideline.J Clin Oncol 2006;2:3187-205.
5.Carlsson G,Ahlin A,Dahllof G,et al.Efficacy and safety of two different rG-CSF preparations in the treatment of patients with severe congenital neutropenia.Br J Haematol 2004;126:127-32.
6.Tarella C,RutellaS,Gualandi F,et al.Consistent bone marrow-derived cell mobilization following repeated short courses of granulocyte–colony-stimulating factor in patients with amyotrophic lateral sclerosis:results from a multicenter prospective trial.Cytotherapy 2010;12:50-9.
7.Zhang Y,Wang L,Fu Y,et al.Preliminary investigation of effect of granulocyte colony stimulating factor on amyotrophic lateral sclerosis.Amyotroph Lateral Scler 2009;10:1-2.
8.Layton JE,Hockman H,Sheridan WP,et al.Evidence for a novelin vivocontrol mechanism of granulopoiesis:mature cell-related control of a regulatory growth factor.Blood 1989;74:1303-7.
9.Zhao LX,Sun JJ,Jiang YP.Application and development of genetic engineering technology in biopharmaceutics.Basic Clin Med 2009;29:996-8.
10.Jiang YP,Jiang W,Dai W,et al.Effect of a mutant human granulocyte colony-stimulating factor,G-CSFC17A-M,on leukopenia in mice and monkeys.Proc Amer Assoc Cancer Res 2005;46:1185-6.
11.Jiang YP,Pannell R,Liu JN,et al.Evidence for a novel binding protein to urokinase-type plasminogen activator in platelet membranes.Blood 1996;87:2775-81.
12.Tanaka H,Okada Y,Kawagishi M,et al.Pharmacokinetics and pharmacodynamics of recombinant human granulocyte-colony stimulating factor after intravenous and subcutaneous administration in the rat.Pharmacol Exp 1989;251:1199-203.
13.Takatani H,Soda H,Fukuda M,et al.Levels of recombinant human granulocyte colony-stimulating factor in serum are inversely correlated with circulating neutrophil counts.Antimicrob Agents Chemother 1996;40:988-91.
 Chinese Medical Sciences Journal2010年1期
Chinese Medical Sciences Journal2010年1期
- Chinese Medical Sciences Journal的其它文章
- Sex Hormones and Androgen Receptor:Risk Factors of Coronary Heart Disease in Elderly Men△
- Comparison between Ophthalmologists and Community Health Workers in Screening of Shallow Anterior Chamber with Oblique Flashlight Test△
- Factors Influencing Pleural Effusion after Fontan Operation:an Analysis with 95 Patients
- Relationship between Carotid Atherosclerosis and Cerebral Infarction
- Expression of FLICE-inhibitory Protein in Synovial Tissue and Its Association with Synovial Inflammation in Juvenile Idiopathic Arthritis△
- A Case of Large“Silent”Extra-adrenal Retroperitoneal Paraganglioma Resected Laparoscopically
