Competition between TRAF2 and TRAF6 Regulates NF-κB Activation in Human B Lymphocytes△
Wen Zhang,Xuan Zhang,Xiao-li Wu,Liu-sheng He,Xiao-feng Zeng,Amrie C.Grammer,and Peter E.Lipsky*
1National Institute of Arthritis and Musculoskeletal and Skin Diseases,Intramural Research Program,National Institutes of Health,Bethesda,MD 20892,USA
2Department of Rheumatology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100730,China
TUMOR necrosis factor (TNF) receptor-associated factor 2 (TRAF2) is a member of TRAF family of adaptor molecules which play important roles in regulating B cell responsiveness.TRAF2 is known to regulate nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) signaling cascades initiated by engagement of TNF-receptor superfamily members such as CD40.1,2Experiments utilizing mice genetically deficient in TRAF2 exhibited gross defects in B cell development as well as rapid postnatal lethality.3Studies utilizing mice transgenic for TRAF domain (TD-TRAF2) exhibited an expanded polyclonal B cell compartment that was resistant to apoptosis,resulting in splenomegaly and lymphadenopathy.4In addition,a variety ofin vitrostudies have examined the role of TRAF2 in B cell responses suggested that Ig secretion was partially dependent on TRAF2-induced TNF and interleukin (IL)-6.5TRAF2 also participated in the synergy between B cell antigen receptor signals and CD40-mediated IgM production.6TRAF2 may play specific roles in mature B cell differentiation and function.However,the role of TRAF2 in CD40-mediated activation of NF-κB is unclear.CD40 engagement on lymph node B cells from mice with TRAF2 conditionally deleted in mature B cells gave mixed results with some NF-κB subunits unaffected and others decreased.7Whereas other experiments with a variety of cell lines indicate that TRAF2 either inhibits or is essential for CD40-induced NF-κB activation.7,8
Another member of the TRAF family,TRAF6,also contributes to CD40-mediated B cell activation.TRAF6 deficient murine splenocytes manifest a deficiency in CD40-mediated activation,9and also a marked decrease in B cell numbers.10In additon,recent evidence indicates that TRAF2 has minimal capacity to activate c-Jun N-terminal kinase (JNK) or increase CD80 expression in the absence of TRAF6.11
The relative roles of TRAF2 and TRAF6 in B cell signaling remain unclear.Moreover,human TRAF6 is both structurally and functionally different in mice and humans.Because of a unique sequence of amino acids present in human,expression of human TRAF6 is more prone to induce apoptosis and not NF-κB activation in a variety of cells.12As a result of this aspect of human TRAF6,the relative roles of TRAF2 and TRAF6 in the activation of human B cells are even more problematic.In this study,we investigated the role of TRAF2 in activation of NF-κB and AP-1 signaling pathways in Ramos B cells,as well as the effect of TRAF2 and TRAF6 on CD40-mediated NF-κB activation.
MATERIALS AND METHODS
Plasmids
Plasmids that encode yellow fluorescent protein (YFP)-fused to wildtype (WT) TRAF2 or the TRAF domains of TRAF2 and TRAF 6 (dominant negative,DN;TRAF domain,TD,lacking the N-terminal RING and zinc finger domains)have been previously described (TR2-TD,272-501;TR6-TD).13,14The plasmids expressing shRNA for TRAF2 or TRAF6 were created by cloning into the IMG800-1 vector(IMGENEX,San Diego,CA,USA).Two shRNAs were created for each TRAF by annealing and cloning each pair of primers into the vector.For TRAF2,TR2-shRNA#1 was specific for nucleotides 1166-1187 (5'-tcgagatgtgtctgcgtatctacctggaattcgaggtagatacgcagacacatcttttt-3';5'-ctagaaaaagatgtgtctgcgtatctacctcgaattccaggtagatacgcagacacatc-3') and TR2-shRNA#2 was specific for nucleotides 1280-1301 (5'-tcgaccagaaggtgaccttaatgctggaattcgagcattaaggtcaccttctggttttt-3';5'-ctagaaaaaccagaaggtgaccttaatgctcgaattccagcattaaggtcaccttctgg-3').For FRET experiments,previously described YFP-TRAF2 and YFP-TRAF6TD plasmids were employed.13Human CD40 was inserted into BalII and HindII sites of the pECFP-N1 vector (Invitrogen,Carlsbad,CA,USA) to generate CD40-CFP constructs.CD40 mutants at either of the TRAF2-binding sites or the TRAF6-binding site were generated with a single site mutation kit (Stratagene,Cedar Creek,TX,USA) following the manufacturer’s instructions.The second TRAF2-binding site was described previously.15
Cell culture,plasmid transfection,and selection of transfected cells
The Ramos/R2G6 EBV-negative B cell line was cultured as previously described.16For transient transfection,Ramos B cells were suspended at a concentration of 1×106/mL in phosphate-buffered saline (PBS),separated into 15 mL aliquots and centrifuged.The supernatant was removed and the cells were resuspended in 100 μL of Nucleofactor Kit T.Plasmid (15-17.5 μg/15×106cells) was added to each tube and run on the O16 program of the AMAXA Nucleofactor Machine (AMAXA,Gaithersburg,MD,USA).Transfected cells were cultured overnight in 12-well plates at 37oC in a humidified atmosphere of 5% CO2at a concentration of 106/mL in RPMI medium supplemented with 10% fetal bovine serum (FBS),penicillin G (200 U/mL),and gentamicin (10 μg/mL).Cells transfected with YFPtagged proteins were sorted for YFP-positive and -negative populations.Cells transfected with plasmids expressing shRNAs were selected by changing the medium containing 1 mg/mL G418 for an additional 48-72 hours.In some cases,CD40 was engaged with a fusion protein of the extracellular domain of human CD154 fused to murine CD8(0.125 μg/1×105cells;hCD154-mCD8α;Ancell,Bayport,MN,USA).For analysis of IκBs,cells were pre-incubated with 10 μg/mL cycloheximide to prevent new protein synthesis before lysis.
Immunoblotting
After selection of transfected cells,whole cell lysates were prepared by incubation for one hour in a buffer containing 20 mmol/L HEPES (pH 7.9),20% Glycerol,1% Nonidet P-40,1 mmol/L MgCl2,0.5 mmol/L EDTA,0.1 mmol/L EGTA,1 mmol/L DTT,1 mmol/L PMSF,and a proteinase inhibitor cocktail (BD Biosciences,Franklin Lakes,NJ,USA).Lysates were clarified by centrifugation at 10 000×gfor 30 minutes at 4°C.Equivalent amounts of cell extracts quantitated using a standard protein assay (BSA curve,read at OD 595 nm,Bio-rad assay) were separated by 4%-12%SDS-PAGE (premade gels from Invitrogen) and assayed for signaling components by immunoblotting using standard conditions.Primary antibodies were used with specificity for NF-κB components:IκBα (BD Biosciences,Franklin Lakes,NJ,USA);pSer32,36IκBα (Cell Signaling,Beverly,MA,USA);IκBβ (Santa Cruz Biotechnologies,Santa Cruz,CA,USA);IκBε(Transduction Laboratories,Franklin Lakes,NJ,USA);p65 (Santa Cruz Biotechnologies);pSer536-p65 (Cell Signaling);IKKα (BD Biosciences);IKKβ (BD Biosciences);IKKδ/TBK1 (IMGENEX,San Diego,CA,USA);IKKi/ε(IMGENEX);CD40,TRAF1,TRAF2,TRAF3,TRAF5,and TRAF6 (Santa Cruz Biotechnologies);MAPKs (JNK,pJNK,ERK,pERK,p38,and p-p38,Santa Cruz Biotechnologies).Secondary antibodies were goat anti-mouse IgG1,goat anti-mouse IgG2a,goat anti-mouse IgM,and goat antirabbit IgG.
DNA binding of NF-κB and AP-1 components
DNA binding of NF-κB (p50,p65,c-Rel) and AP-1 (c-fos,c-jun,CREB,ATF2) components was examined in nuclear lysates by the enzyme-linked immunosorbent assay(ELISA)-based TransFactor Assay.Nuclear extracts were prepared by using TransFactor extraction kit (BD biosciences).Briefly,cells were incubated in hypotonic pre-lysis buffer (10 mmol/L HEPES at pH 7.9,1.5 mmol/L MgCl2,10 mmol/Lol/L KCl,1 mmol/L DTT,protease inhibitor cocktail) for 15 minutes followed by disruption with rapid strokes with a narrow-gauge needle syringe (23GA)and centrifugation at 10 000×gfor 20 minutes to isolate nuclei. The pellet was resuspended in nuclear extraction buffer (20 mmol/L HEPES at pH 7.9,1.5 mmol/L MgCl2,0.42 mol/L NaCl,0.2 mmol/L EDTA,25% glycerol,1 mmol/L DTT,1 mmol/L PMSF,and protease inhibitor cocktail) and nuclei were disrupted with rapid strokes by a 23GA syringe.The nuclear suspension was shaken gently for 30 minutes at 4°C followed by centrifugation at 20 000×gfor 10 minutes at 4°C.Equivalent amounts of nuclear lysates were loaded into TransFactor plates that are precoated with oligonucleotides containing consensus sequences for NF-κB and AP-1 components and incubated for 60 minutes at 20°C,bound transcription factors were detected with specific primary antibodies for 60 minutes,followed by washing with Transfactor Buffer and then detection with horse radish peroxidase (HRP)-conjugated secondary antibody followed by developing with tetramethyl benzidine (TMB) substrate.Resultswere measured with a microtiter ELISA plate reader at OD 655 nm.
Immunoprecipitation
Cells were lysed in co-immunoprecipitation buffer (NP-40:50 mmol/L Tris-Hcl at pH 7.4,150 mmol/L NaCl,0.5% NP-40,1 mmol/L EDTA,20 mmol/L beta-glycerophosphate,1 mmol/L Na3VO4,1 mmol/L NaF,1 mmol/L PMSF,and complete protease inhibitor cocktail;RIPA Buffer:50 mmol/L Tris-HCl,150 mmol/L NaCl,1 mmol/L PMSF,1 mmol/L EDTA,5 μg/mL aprotinin,5 μg/mL leupeptin,1% Triton X-100,1%sodium deoxycholate,0.1% SDS) for 30 minutes at 4°C.Extracts were pre-cleared with protein G-sepharose beads(Amersham Biosciences,Piscataway,NJ,USA) for 1 hour at 4°C with rotation and then incubated with antibodies of interest and protein G-sepharose beads overnight at 4°C with rotation.For control,the same amounts of cell lysates were incubated with control rabbit or mouse Ig and protein G-sepharose beads at 4°C with rotation overnight.Immunoprecipitates were then washed three times in coimmunoprecipitation buffer before boiling in SDS-sample buffer and analyzing by SDS-PAGE/immunoblotting.
IKK activity assay
Immunoprecipitates for specific IKK family members were washed four times in co-immunoprecipitation buffer followed by two subsequent washes in kinase buffer (25 mmol/L Hepes,pH 7.6,25 mmol/L beta-glycerophosphate,15 mmol/L MgCl2,1 mmol/L DTT,0.5 mmol/L Na3VO4,0.5 mmol/L NaF).Immunoprecipitates were then incubated for 30 minutes at 30°C with kinase buffer containing 5 μCi[γ-32P]-ATP and 2 μg GST-IκBα before protein fractionation by SDS-PAGE and autoradiography.
Fluorescence resonance energy transfer (FRET)
Hela cells were co-transfected with 500 ng each of YFPTRAF2 and wild type CFP-CD40 or a CFP-CD40-construct mutated at the classic TRAF2-binding site (T254A) or mutated at both known TRAF2-binding sites (T254A and 272SVQE->AVQA) or a CFP-CD40 mutant mutated at the TRAF6-binding site (231QEPQE->QEGQA).After overnight incubation,these co-transfected cells were analyzed for CFP→YFP FRET signals using a Dako Cyan flow cytometer as described.17
Statistical analysis
Data were shown as mean±standard error of the mean(SEM) and were tested for statistical significance using the Student’sttest.A value ofP<0.05 was considered statistical significance.
RESULTS
Verification of transfection of Ramos B cells with TRAF2 plasmids
The plasmids expressing YFP,YFP-WT-TRAF2,and YFP-TDTRAF2 as well as shRNA specific for TRAF2 silencing were prepared and transiently expressed in Ramos B cells.For the YFP constructs,YFP-positive cells were selected by flow cytometry with a post-sort purity of greater than 97% (Fig.1A).YFP-TRAF2 constructs,both WT and TD,of the expected molecular weights were readily detected in the post-sort populations (Fig.1B).Endogenous TRAF2 and CD40 expression were not significantly affected by overexpression of the YFP-WT-TRAF2 or YFP-TD-TRAF2 construct (Fig.1B).Expression of TRAF2-shRNA decreased endogenous TRAF2 expression by more than 50% but did not consistently and significantly affect the amounts of CD40 or TRAFs-3,-5,and -6 in Ramos B cells (Fig.1C).
Alteration of TRAF2 expression in Ramos B cells affected p38 phosphorylation,ERK phosphorylation,and nuclear translocation of active c-fos

Figure 1.Verification of transfection of Ramos B cells with TRAF2 plasmids.
To confirm that the TRAF2 constructs were active,the phosphorylation status of ERK,JNK,and p38 as well as nuclear translocation and DNA binding of c-fos,c-jun,CREB,and ATF2 were analyzed by immunoblotting (Fig.2A)and an ELISA-based DNA binding assay (Fig.2B).Overexpression of YFP-TRAF2 significantly increased the ratio of phosphorylated to total MAPK for ERK and p38,but not for JNK,as well as nuclear translocation and DNA binding of c-fos,but not c-jun,CREB,and ATF2.Constitutive activity of MAPKs in Ramos cells was dependent on TRAF2 activity.Thus,decreasing TRAF2 function accomplished by RNA silencing with TRAF2-shRNA or expression of the TD-TRAF2 mutant decreased the ratio of phosphorylated to total MAPK for ERK and p38,but not for JNK,as well as nuclear translocation and DNA binding of c-fos,but not c-jun,CREB,and ATF2.We also noted that expression of TRAF2 shRNA decreased expression of p38 and JNK1,whereas overexpression of TD-TRAF2 did not.
Alteration of TRAF2 expression in Ramos B cells affected specific aspects of the NF-κB pathway
The role of TRAF2 in signaling mechanisms involved in the canonical NF-κB pathway was examined next.As shown in figure 3A,phosphorylation of IκBα at Ser32,36was induced by YFP-WT-TRAF2 and inhibited by YFP-TD-TRAF2 or TRAF2-shRNA.Notably,however,whereas YFP-WT-TRAF2 induced degradation of IκBα (0.63 ± 0.08,compared with control,n=4),silencing TRAF2 RNA (#1:0.98 ± 0.03;#2:0.87 ± 0.06) or only expressing the TD of TRAF2 had no consistent effect on IκBα degradation (1.20 ± 0.26).In addition,manipulation of TRAF2 in Ramos B cells had no effect on total levels of the other IκB family members,IκB-β,and IκB-ε (Fig.3A).
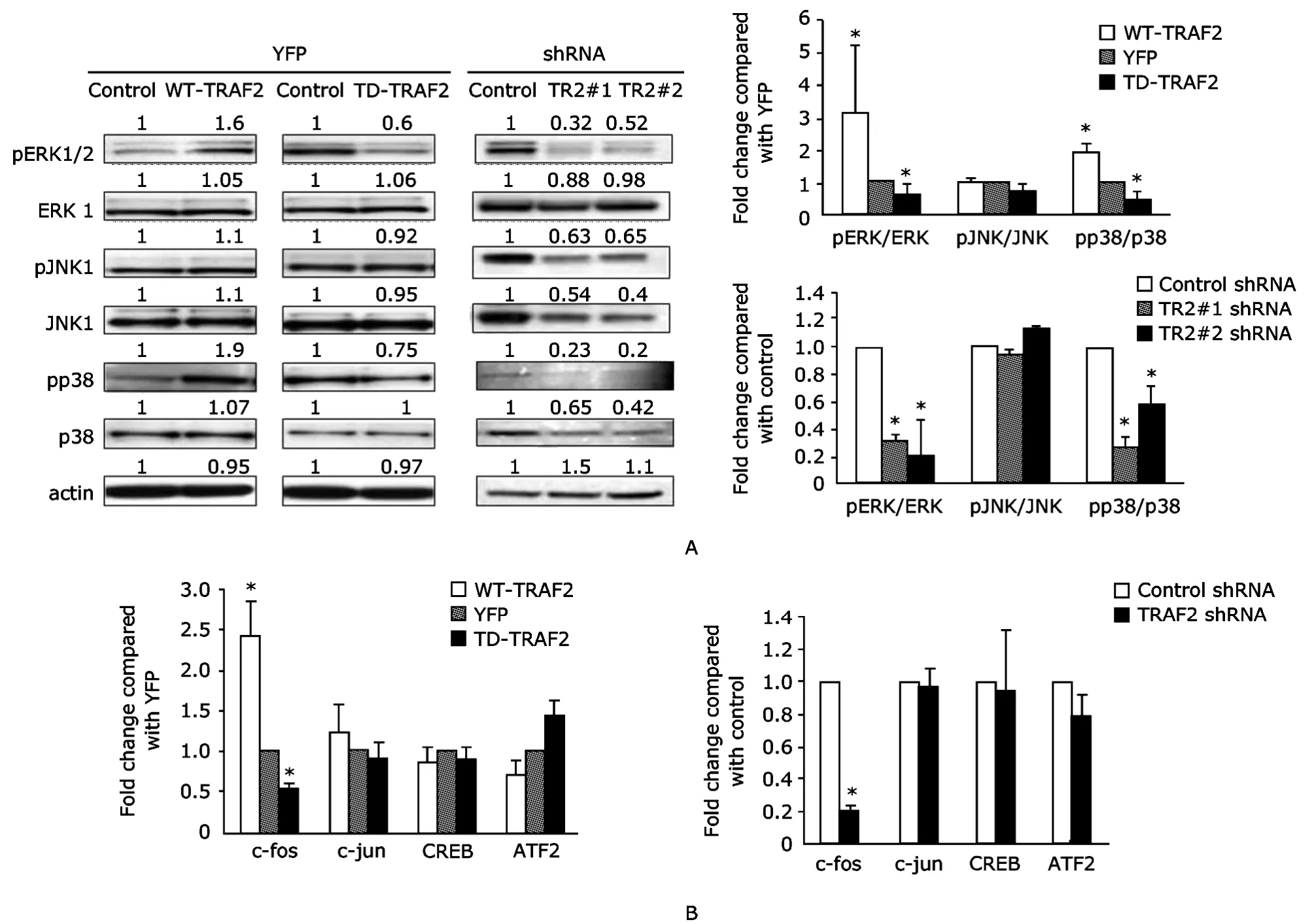
Figure 2. Alteration of TRAF2 in Ramos B cells affects p38 phosphorylation,ERK phosphorylation,and nuclear translocation of c-fos.
Alteration of TRAF2 expression in Ramos B cells also had a consistent affect on p65 phosphorylation (Ser536) and nuclear translocation of active p65.Specifically,results showed that total levels of p65 did not change with TRAF2 manipulation,whereas p65 phosphorylated at Ser536was increased with YFP-WT-TRAF2 and decreased with YFP-TDTRAF2 or TRAF2-shRNA (Figs.3B-D).In addition,overexpression of YFP-WT-TRAF2 consistently increased nuclear translocation and DNA binding of p65,p50,and c-Rel(Fig.3E).In contrast,decreasing TRAF2 function with the YFP-TD-TRAF2 construct or TRAF2 shRNA consistently affected nuclear translocation and DNA binding of p65(Fig.3E),but not p50 or c-Rel.
To determine the upstream IKK family members utilized by TRAF2 in Ramos B cells,anin vitrokinase assay was employed.Although overexpression of YFP-WT-TRAF2 increased TRAF2 immunoprecipitation of all IKK family members (Fig.4B),YFP-WT-TRAF2 specifically increased catalytic kinase activity of IKK-α and–i/ε,but not IKK-β or IKK-δ/TBK1,for the substrate GST-IκBα (Fig.4A).
Decreased TRAF6 expression or activity in Ramos B cells inhibited the canonical NF-κB pathway
To examine the role of TRAF6 in constitutive NF-κB activity in Ramos B cells,dominant negative TRAF6 (TD-TRAF6)and shRNA to TRAF6 was employed.Silencing of endogenous TRAF6 with TRAF6-shRNA (Fig.5A) decreased phosphorylation of IκBα and p65 (Ser536) in Ramos B cells(Fig.5B),as well as inhibited nuclear translocation and DNA binding of all three NF-κB transcription factors examined (Fig.5C).Similarly, expression of TD-TRAF6 decreased phosphorylation of IκBα and p65 (Fig.5B).
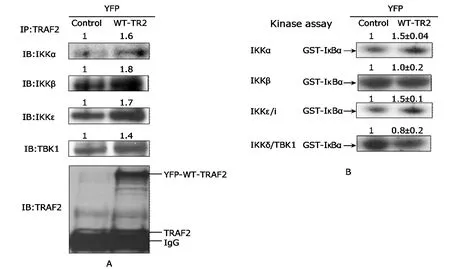
Figure 4.TRAF2 induces kinase activity of IKKs-α and -i/ε in Ramos B cells.
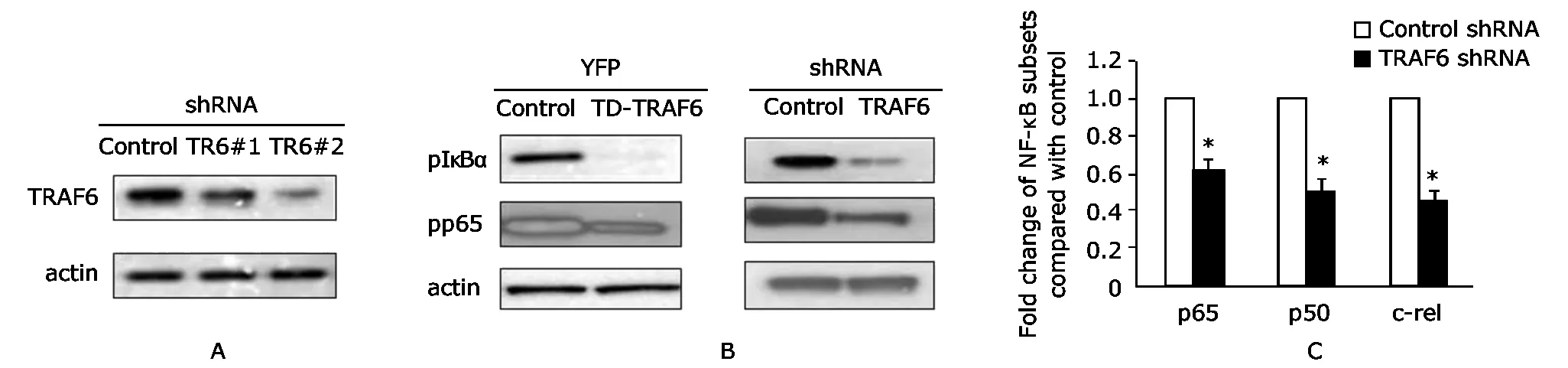
Figure 5.Decreased expression of TRAF6 inhibits constitutive NF-κB pathway activity.
Stimulation of recombinant CD154 induced binding of TRAF2 and TRAF6 with CD40
To address the comparative roles of TRAF2 and TRAF6 in NF-κB activation,we transiently transfected Ramos cells with an NF-κB reporter construct along with vectors expressing WT versions of TRAFs 1,2,3,5,and 6.Analysis of luciferase activity indicated that WT-TRAF6 increased NF-κB activity significantly more than the other TRAFs(P<0.05) (Fig.6A).Overexpression of WT-TRAF2 also increased NF-kB luciferase activity significantly (P<0.05),but significantly less than TRAF6 (P<0.01) (Fig.6A).We next determined the TRAFs that interacted with CD40 after engagement of CD40 by CD154.Stimulation of Ramos B cells with recombinant CD154 induced direct association of CD40 with TRAF2 and TRAF6,but not other TRAF family members (Fig.6B).
Impact of TRAF2 and TRAF6 on CD40-mediated activation of NF-kB
Engagement of CD40 by recombinant CD154 induced phosphorylation of IκBα and p65,and degradation of IκBα(Fig.7).Notably,phosphorylation of IκBα and p65 following CD40 engagement was augmented in cells with TRAF2-shRNA (Fig.7A).In contrast,phosphorylation of IκBα was significantly suppressed by overexpression of TD-TRAF2,whereas phosphorylation of p65 was not significantly affected (Fig.7B).Similar results were noted when nuclear translocation and DNA binding of p65 were examined.Thus,overexpression of WT-TRAF2 suppressed,whereas TRAF2 shRNA augmented and overexpression of TD-TRAF2 had no effect on p65 nuclear translocation and DNA binding (Figs.7C,D).In contrast to the effects noted with modulation of TRAF2 expression,decreased TRAF6 function either following silencing with TRAF6-shRNA or overexpression of YFP-TD-TRAF6 inhibited CD40-mediated phosphorylation of IκBα (Figs.7E,F),degradation of IκBα(Fig.7F),and nuclear translocation and DNA binding of p65(Figs.7C,D).
Competition between TRAF2 and TRAF6 for binding to CD40
By using CFP→YFP FRET,interaction between CD40 and TRAF2 or TRAF6 was detected.Interaction between CD40 and TRAF2 was greatly diminished by mutation of the classic TRAF2 binding site on the cytoplasmic tail of CD40(250PVQET,CFP-CD40T2m-T254A) and nearly completely eliminated when both known binding sites were mutated(CFP-CD40T2m) (Fig.8A).Similarly,the binding of TRAF6 to CD40 was also abolished when its binding site was mutated (Fig.8B).Notably,binding of TRAF2 to CD40 was increased when the TRAF6 binding site was mutated (Fig.8A),whereas the binding of TRAF6 to CD40 was enhanced when the TRAF2 binding sites were mutated (Fig.8B).
DISCUSSION
The experiments utilizing a human B cell line document that TRAF2 can play roles in both NF-κB and AP-1 signaling pathways.Thus,TRAF2 specifically induced phosphorylation of IκBα (Ser32,36) as well as nuclear translocation and phosphorylation of p65 in the NF-κB pathway and also phosphorylation of ERK and p38,as well as nuclear translocation of c-fos.In addition,although TRAF2 associated with all IKK family members,it specifically induced activation of IKKα and IKKi/ε.These results are consistent with previous findings demonstrating that IKKα mediates the action of upstream activators of p65,including TRAF2,RIP,and NIK.18-20
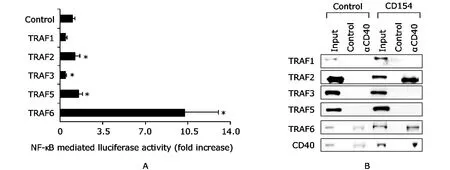
Figure 6.Stimulation of recombinant CD154 induces binding of TRAF2 and TRAF6 with CD40.
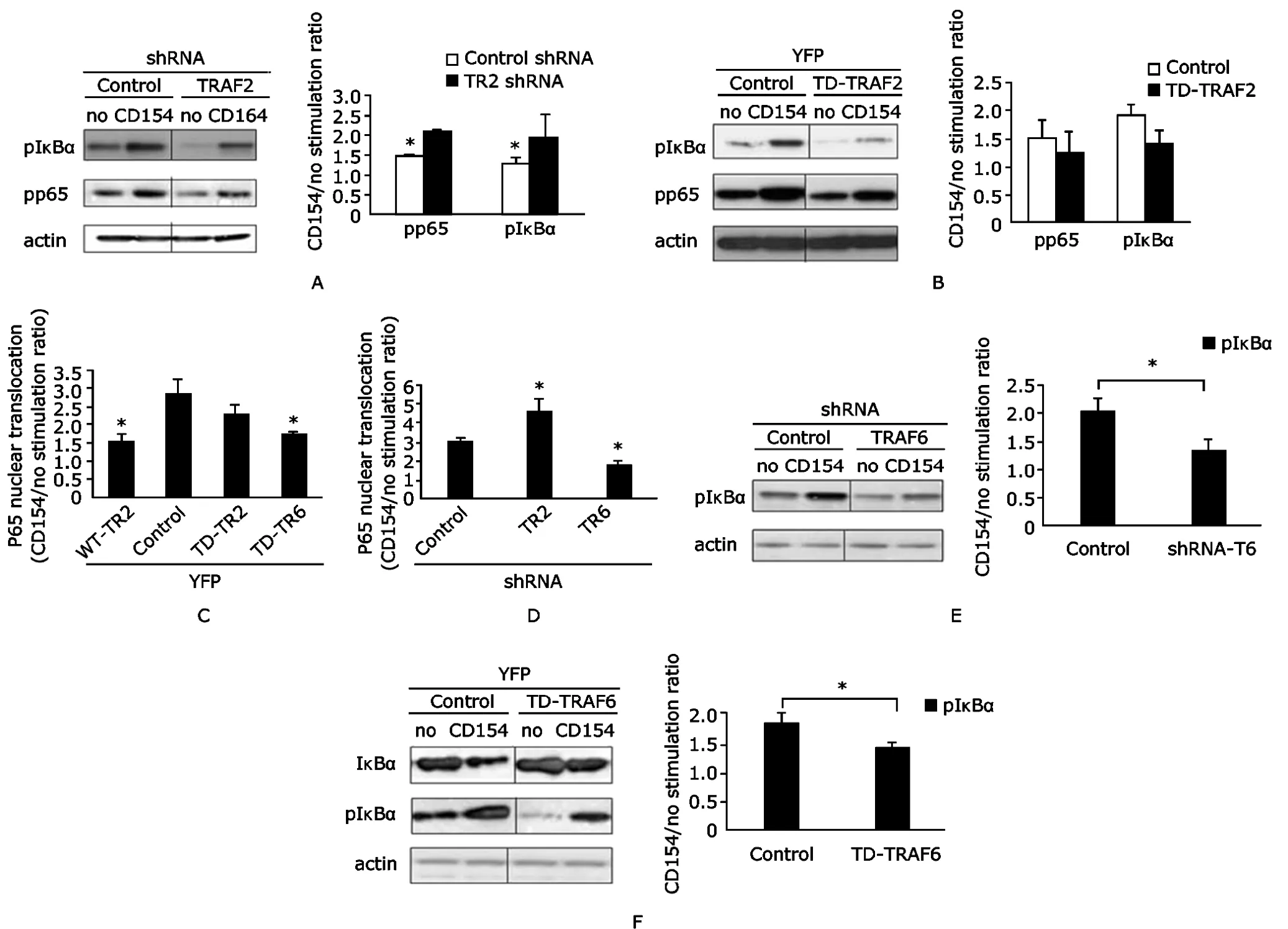
Figure 7.TRAF2 negatively regulates and TRAF6 enhances CD40-mediated activation of NF-κB.
In the Ramos B cell line,nuclear translocation and DNA binding of the NF-κB subunit p65,but neither p50 nor c-rel,was dependent upon TRAF2 as demonstrated with the TD-TRAF2 construct or by RNA silencing with TRAF2-shRNA.By contrast,overexpression of TRAF2 induced nuclear translocation and DNA binding of all the canonical NF-κB subunits (p50,p65,c-Rel).Together,these results indicate that TRAF2 can play a role in,but is not essential for,the constitutive p50 and c-Rel mediated pathways in Ramos cells.Further experiments demonstrated a role for TRAF6 in constitutive nuclear translocation and DNA binding of all canonical NF-κB subunits since TD-TRAF6 or TRAF6-shRNA resulted in decreased nuclear translocation and DNA binding of p50,p65,and c-Rel.The multifaceted role of TRAF6 in NF-κB activation was recently clarified by a study indicating that TRAF6 can function together with and downstream of TRAF2 to activate canonical NF-κB subunits.21In conjunction with the data presented here,these results suggest that nuclear translocation and DNA binding of p50 and c-Rel induced by overexpression of YFP-WT-TRAF2 may be partially mediated by signaling involving TRAF6.
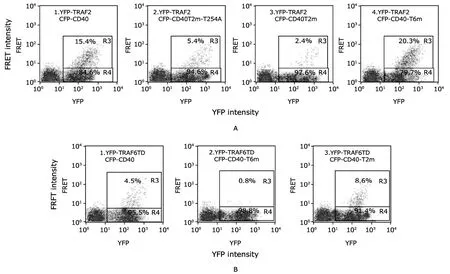
Figure 8.Flow cytometric profiles of FRET analysis between human wild type and mutant CD40 and TRAF2 or TRAF6 indicate that there is competition between TRAFs for CD40 binding.
Studies of the role of TRAF family members in CD40 signaling have revealed species and cell type differences.12,22,23The role of TRAF2in activation of NF-κB by TNFR family members has beenparticularly unresolved.Early studies in epithelial cells suggestedthat TRAF2 is essential to this function.24However,later experimentsshowed that NF-κB activation in B cells by CD40 with defective TRAF2 binding is only slightly less than thatstimulated by WT-CD40.25In our studies,overexpression of WT-TRAF2 increased NF-κB activity,but not as strongly as overexpression of TRAF6.In addition,overexpression of TD-TRAF2 in human B cell lines partially decreased phosphorylation of IκBα and p65,as well as nuclear translocation of p65,suggesting that TRAF2 has potent signaling capability in B cells.However,TRAF2 appeared to play only a modest positive role in CD40-induced NF-κB activation.Rather,the presence of TRAF2 exerted a negative influence on CD40-induced NF-κB activation.
Similarly,the mechanisms by which TRAF6 contribute to CD40-induced NF-κB activation are not entirely clear.Consistent with our data,which demonstrated that in Ramos B cells TRAF6 plays the dominant role in CD154-stimulated activation of NF-κB,others have found that cells expressing the CD40 N237D receptor with increased TRAF6 binding exhibited the highest basal and CD154-stimulated levels of NF-κB activation.26In contrast,it has also been reported that disruption of the TRAF6 binding site in CD40 did not markedly interfere with the ability of CD40 to activate NF-κB.6The current studies may begin to resolve these apparent inconsistencies by suggesting that the interplay of TRAF6 and TRAF2,rather than the action of individual TRAFs in isolation,may determine the overall capacity of CD40 engagement to activate NF-κB.
TRAF2 and TRAF6 have unique and overlapping activities in CD40 signaling,11suggesting that there is a complex interplay between TRAF2 and TRAF6 in the regulation of CD40 signaling.Whereas TRAF6 appears to play the dominant role in CD40-induced activation of NF-κB in human B cells,it is clear that CD40-induced IκBα phosphorylation and degradation are partially TRAF2 dependent.6,7Previous studies have also shown that TRAF2 might participate in recruitment of TRAF6 to CD40,27although a TRAF6 mutant(TRAF6T471A) can be recruited to CD40 without TRAF2.16The current data add a new possible explanation for the regulatory role of TRAF2 in modulating CD40-induced NF-κB activation in human B cells,namely competition for CD40 binding.Our data showed that stimulation of Ramos B cells with recombinant CD154 induces direct association of TRAF2 and TRAF6,but not other TRAF family members,with CD40.Using FRET,we were able to show that mutation of the TRAF2 binding sites on CD40 increased TRAF6 binding,whereas deleting the TRAF6 binding site enhanced TRAF2 binding.These data are consistent with the conclusion that TRAF6 and TRAF2 compete for binding to CD40,even though each has a unique binding site.These results suggest that competition for CD40 binding between TRAF2 and TRAF6 may play a novel role in determining the outcome of CD40 signaling.
In summary,the current data define the potential role for TRAF2 and TRAF6 in human B cell signaling.In the Ramos B cell line,TRAF2 and TRAF6 are involved in discrete sets of constitutive signaling cascades.Following CD154 stimulation of Ramos B cells,TRAF6 appears to play the major role in activating NF-κB,whereas TRAF2 becomes largely inhibitory of CD40-mediated nuclear translocation and activation of p65 possibly by competing with TRAF6 for binding to CD40.These findings provide new insight into the potential regulation of NF-κB signaling,both constitutively and after CD40 engagement in human B cells by suggesting that the outcome of signaling is influenced by the availability of TRAF2 and TRAF6 and their competitive interactions with CD40.Examination of the relative effects of TRAF2 and TRAF6 in primary human B cells that alter expression of these adaptor molecules with activation and differentiation will be important in confirming their respective roles during induction of human humoral immune responses.
1.Xia ZP,Chen ZJ.TRAF2:a double-edged sword? Sci STKE.2005;2005:pe7.
2.Bouwmeester T,Bauch A,Ruffner H,et al.A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway.Nat Cell Biol 2004;6:97-105.
3.Cannons JL,Bertram EM,Watts TH.Cutting edge:profound defect in T cell responses in TNF receptor-associated factor 2 dominant negative mice.J Immunol 2002;169:2828-31.
4.Lee SY,Reichlin A,Santana A,et al.TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival.Immunity 1997;7:703-13.
5.Hostager BS,Bishop GA.Role of TNF receptor-associated factor 2 in the activation of IgM secretion by CD40 and CD120b.J Immunol 2002;168:3318-22.
6.Hostager BS,Haxhinasto SA,Rowland SL,et al.Tumor necrosis factor receptor-associated factor 2(TRAF2)-deficient B lymphocytes reveal novel roles for TRAF2 in CD40 signaling.J Biol Chem 2003;278:45382-90.
7.Grech AP,Amesbury M,Chan T,et al.TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells.Immunity 2004;21:629-42.
8.Carpentier L,Declercq W,Malinin NL,et al.TRAF2 plays a dual role in NF-kappaB-dependent gene activation by mediating the TNF induced activation of p38 MAPK and IkappaB kinase pathways.FEBS Lett 1998;425:195-8.
9.Lomaga MA,Yeh WC,Sarosi I,et al.TRAF6 deficiency results in osteopetrosis and defective interleukin-1,CD40,and LPS signaling.Genes Dev 1999;13:1015-24.
10.Naito A,Azuma S,Tanaka S,et al.Severe osteopetrosis,defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice.Genes Dev 1999;4:353-62.
11.Rowland SL,Tremblay MM,Ellison JM,et al.A novel mechanism for TNFR-associated factor 6-dependent CD40 signaling.J Immunol 2007;179:4645-53.
12.He L,Wu X,Siegel R,et al.TRAF6 regulates cell fate decisions by inducing caspase 8-dependent apoptosis and the activation of NF-kappaB.J Biol Chem 2006;281:11235-49.
13.He L,Olson DP,Wu X,et al.A flow cytometric method to detect protein-protein interaction in living cells by directly visualizing donor fluorophore quenching during CFP-->YFP fluorescence resonance energy transfer (FRET).Cytometry A 2003;55:71-85.
14.Pullen SS,Dang TT,Crute JJ,et al.CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs).Binding site specificity and activation of downstream pathways by distinct TRAFs.J Biol Chem 1999;274:14246-54.
15.Lu LF,Cook WJ,Lin LL,et al.CD40 signaling through a newly identified tumor necrosis factor receptor-associated factor 2 (TRAF2) binding site.J Biol Chem 2003;278:45414-8.
16.He L,Grammer AC,Wu X,et al.TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF-B activation.J Biol Chem 2004;279:55855-65.
17.He L,Wu X,Simone J,et al.Determination of tumor necrosis factor receptor-associated factor trimerization in living cells by CFP->YFP->mRFP FRET detected by flow cytometry.Nucleic Acids Res 2005;33(6):e61.
18.Asamitsu K,Tetsuka T,Kanazawa S,et al.RING finger protein AO7 supports NF-κB-mediated transcription by interacting with the transactivation domain of the p65 subunit.J Biol Chem 2003;278:26879-87.
19.Sakurai H,Suzuki S,Kawasaki N,et al.Tumor necrosis factor-induced IKK phosphorylation of NF-κB p65 on Serine 536 is mediated through the TRAF2,TRAF5,and TAK1 signaling pathway.J Biol Chem 2003;278:36916-23.
20.Mattioli I,Sebald A,Bucher C,et al.Transient and selective NF-κB p65 Serine 536 phosphorylation induced by T cell costimulation is mediated by IB kinase and controls the kinetics of p65 nuclear import.J Immunol 2004;172:6336-44.
21.Davies CC,Mak TW,Young LS,et al.TRAF6 is required for TRAF2-dependent CD40 signal transduction in nonhemopoietic cells.Mol Cell Biol 2005;25:9806-19.
22.Bishop GA,Hostager BS.Molecular mechanisms of CD40 signaling.Arch Immunol Ther Exp (Warsz) 2001;49:129-37.
23.Quezada SA,Jarvinen LZ,Lind EF,et al.CD40/CD154 interactions at the interface of tolerance and immunity.Annu Rev Immunol 2004;22:307-28.
24.Rothe M,Sarma V,Dixit VM,et al.TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40.Science 1995;269:1424-7.
25.Hsing Y,Hostager BS,Bishop GA.Characterization of CD40 signaling determinants regulating nuclear factor-kappa B activation in B lymphocytes.J Immunol 1997;159:4898-906.
26.Mann J,Oakley F,Johnson PW,et al.CD40 induces interleukin-6 gene transcription in dendritic cells:regulation by TRAF2,AP-1,NF-B,and CBF1.J Biol Chem 2002;277:17125-38.
27.Lee EG,Boone DL,Chai S,et al.Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice.Science 2000;289:2350-4.
 Chinese Medical Sciences Journal2010年1期
Chinese Medical Sciences Journal2010年1期
- Chinese Medical Sciences Journal的其它文章
- Sex Hormones and Androgen Receptor:Risk Factors of Coronary Heart Disease in Elderly Men△
- Comparison between Ophthalmologists and Community Health Workers in Screening of Shallow Anterior Chamber with Oblique Flashlight Test△
- Factors Influencing Pleural Effusion after Fontan Operation:an Analysis with 95 Patients
- Relationship between Carotid Atherosclerosis and Cerebral Infarction
- Expression of FLICE-inhibitory Protein in Synovial Tissue and Its Association with Synovial Inflammation in Juvenile Idiopathic Arthritis△
- A Case of Large“Silent”Extra-adrenal Retroperitoneal Paraganglioma Resected Laparoscopically
