以 2,5-二巯基-1,3,4 噻二唑(Ⅰ)为桥连配体的二维镉(Ⅱ)配合物的制备与晶体结构
王文祥 赵 红
(东南大学化学化工学院有序物质科学研究中心,南京 211189)
以 2,5-二巯基-1,3,4 噻二唑(Ⅰ)为桥连配体的二维镉(Ⅱ)配合物的制备与晶体结构
王文祥 赵 红*
(东南大学化学化工学院有序物质科学研究中心,南京 211189)
镉配合物;水热合成;晶体结构;荧光性质
1,3,4-thiadiazole-2,5-dithiol(CAS No.1072-71-5),BismuthiolⅠ,attracted the attention of researchers because it is a versatile ligand with tautomeric forms[1-2](Scheme 1).A bound of coordination compounds were obtained from the combination of BismuthiolⅠand metal centers,especially main group and transition metals.In some compounds,BismuthiolⅠremains thiol-thiol tautomeric form(Ⅱ),in some it is thione-thiol tautomeric form(Ⅱ),and also it is known to be thionethione tautomeric form(Ⅱ)in some compounds[3-5].
In our particular research,we have combined metal salts with potentially bridging organic ligands under hydrothermal conditions to produce a range of new materials[6-7].The versatility of BismuthiolⅠin coordination compounds give us some ideas to construct the new complexes containing BismuthiolⅠas a bridg-ing ligand under hydrothermal or solvothermal conditions.To the best of our knowledge,such structural studies of BismuthiolⅠto date include a few of transition metals,e.g.,Ru,Pt,Au,Cr,Cu,Zn,et al.[8-17],and main-group Tl and Sn[18-19].None concerning the cadmium(Ⅱ)coordination polymer containing BismuthiolⅠ(thione-thiol tautomeric formⅡ)as a bridging ligand has been reported so far.In this text,we report the synthesis,crystal structure and solid state fluorescence of a 2D network cadmium(Ⅱ)coordination polymer,[Cd(5-thioxo-4,5-dihydro-1,3,4-thiadiazole-2-thiolate)2]n(1).

1 Experimental
1.1 Materials and instruments
Commercially available reagents were used as received without further purification.IR spectra were obtained with KBr pellets in the 4000~400 cm-1region,using a Shimadzu IRprestige-21 spectrophotometer.The crystal structures were determined by Rigacu SCX mini diffractionmeter.Electronic spectrum was recorded on Shimadzu RF-5301pc spectrophotometer.
1.2 Preparation of 1,3,4-thiadiazole-2,5-dithiol
To a mixture of 85%hydrazine hydrate(1 mmol)and triethylamine (4 mg)in KOH (6.7 mg,in 3 mL H2O),carbon disulfide(3.5 mmol)was added dropwise.The mixture was stirred under 5℃about 2 h,then refluxed with stirring for 2.5 h.Dilution with cold water(50 mL)and acidification with concentrated HCl precipitated a yellow solid.The product was filtered,washed with cold water,dried in air,and used for next step without further purification.
1.3 Preparation of[Cd(5-thioxo-4,5-dihydro-1,3,4-thiadiazole-2-thiolate)2]n(1)
CdCl2(1 mmol)and 1,3,4-thiadiazole-2,5-dithiol(2 mmol)were placed in a thick Pyrex tube(ca.20 cm in length).After addition of 2.0 mL of water,the tube was frozen with liquid N2,evacuated under vacuum,and sealed with a torch.The tube was heated at 100 ℃ for 2 days to give colorless prism crystals (pure phase).Yield:52%(based on CdCl2).IR spectrum(cm-1):3163(w),2927(w),1473(s),1417(s),1400(m),1290(w),1 167(m),1 093(m),1 029(s),731(m),636(w),576(w),543(w).
1.4 Crystal data of 1
A colorless crystal of the title compound with dimensions of 0.25 mm ×0.20 mm ×0.12 mm was selected for the X-ray diffraction experiment.Diffraction data were collected with a Rigacu SCX mini diffractionmeter using Mo Kα radiation (λ=0.071 073 nm).The structure was solved by direct methods with SHELXS-97 and refined by full matrix least squares on F2with SHELXL-97.All non-hydrogen atoms were refined with anisotropic thermal parameters.Hydrogen atoms were added theoretically and refined with riding model and fixed isotropicthermalparameters.Detailed data collection and refinement of the compound 1 are summarized in Table 1,and the selected bond distances and angles are listed in Table 2.
CCDC:689817.
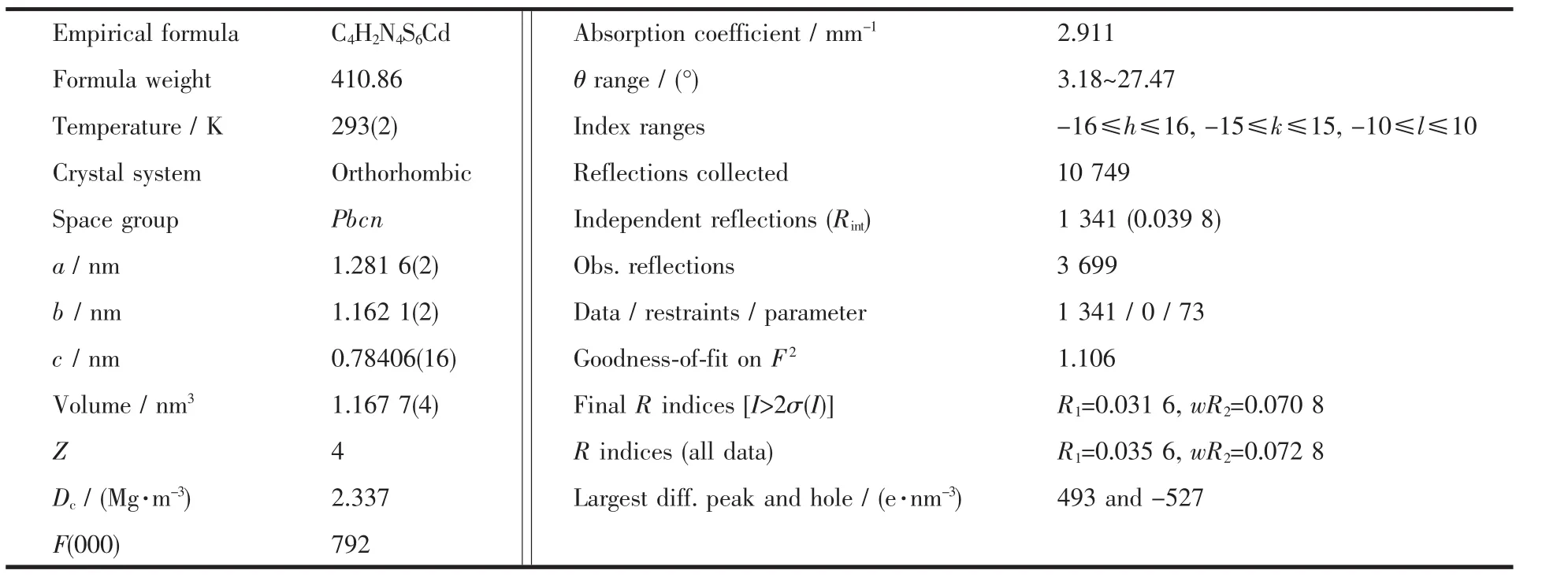
Table 1 Crystallographic data for compound 1

Table 2 Selected bond lengths(nm)and angles(°)for compound 1
2 Results and discussions
HydrothermaltreatmentofBismuthiolⅠandCdCl2in the present of water as solvent at 100℃affords a Cd(Ⅱ)compound 1.Single crystal X-ray crystallographic analysis reveal that the title compound 1 has a twodimensional framework constructed from cadmium ions and μ2-Bismuthiol Ⅰ ligands.An ORTEP drawing of the structure,showing the atom numbering scheme,is given in the Fig.1.Meanwhile,the selected bond distances and angles are reported.In the crystal structure of compound 1,the Cd atom is located at a site of 2 symmetry and the local coordination geometry around the Cd center can be best described as a slightly distorted tetrahedron with four S atoms from four symmetry-related BismuthiolⅠligands,in which the Cd-S distances of 0.252 11(9)and 0.254 50(10)nm are normal and the S-Cd-S angles range from 104.33(6)°to 110.78(4)°.The ligand part of the molecule is practically planar with the maximum atomic deviation fron the least-squares mean plane is 0.064 7(13)for S(3).Interatomic distances follow the same pattern previously observed for the thione-thiol tautomeric form(Ⅱ)compounds[21]:one of the exocyclic C-S bond lengths[C(2)-S(3),0.169 9(3)nm]is significantly shorter than the other[C(1)-S(2),0.172 5(4)nm],suggesting different amounts of double bond character in these two bonds.Correspondingly,the two cyclic C-N distances are markedly different,C(1)-N(1)[0.129 6(4)nm]being an almost pure double bond.
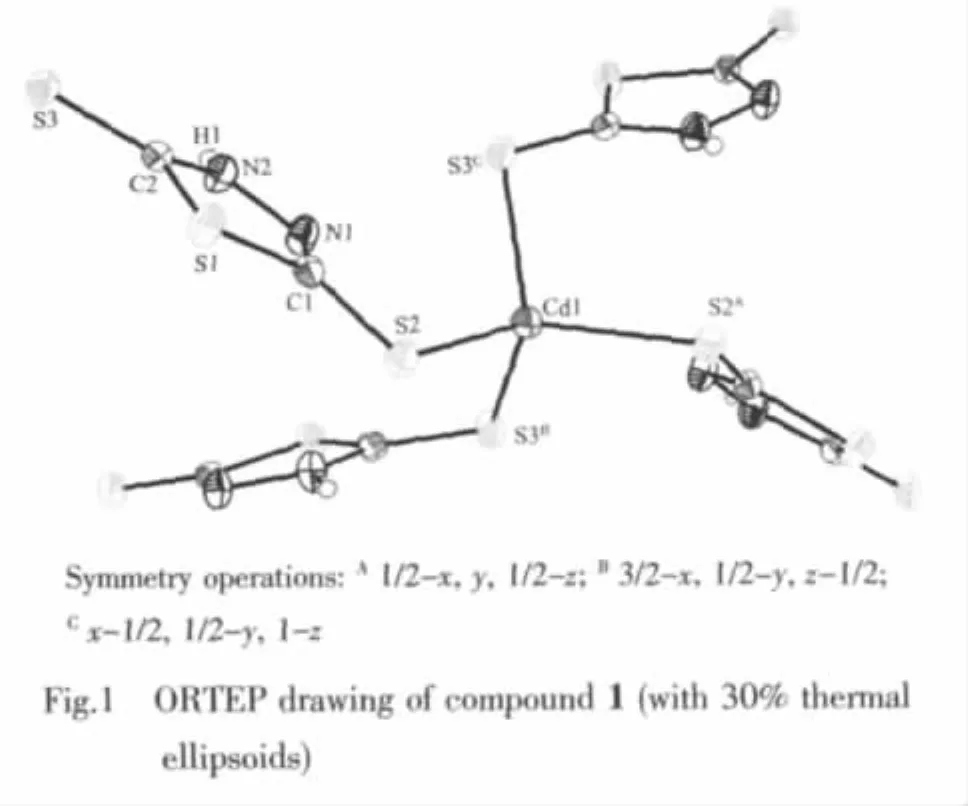
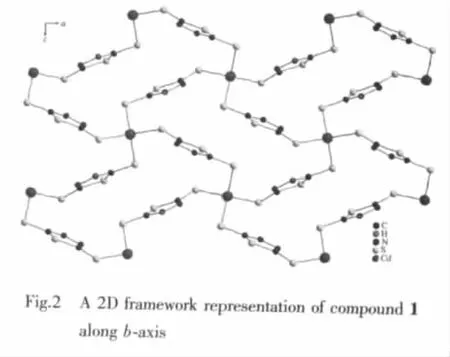
As showing in Fig.2,each of the 5-thioxo-4,5-dihydro-1,3,4-thiadiazole-2-thiolate ligands bridges two Cd atoms through two terminal S atoms,leading to the formation of a chiral two-dimensional layer contain homochiral helical chains (left-or right-hand single helical chain).The separation of Cd centers across the ligands is 0.792 7(1)nm and the screw-pitch is 0.784 1(2)nm(Fig.3).However,it is worth noting that the adjacent layers have opposite chirality,and were connected into a three-dimensional framework through N-H…S hydrogen bonds[H…S 0.265(5)nm]Finally,thesolid statefluorescentemission spectrum of complex 1 at room temperature shows that maximum emission peak occurs in ca.414 nm (λex=368 nm)(Fig.4).The photoluminescent mechanism may be considered ligand to ligand transition.


In summary,a two-dimensionalcoordination network was afforded where Cd centers are linked by BismutiolⅠwith thione-thiol tautomeric form.The result gives great encouragement to find more new functional coordination polymers.
[1]Emeleus H J,Hass A,Sheppard N.J.Chem.Soc.,1963:3168-3171
[2]Thorn G D.Can.J.Chem.,1960,38:1439-1444
[3]Raper E S.Corrd.Chem.Rev.,1985,61:115-184
[4]Raper E S.Corrd.Chem.Rev.,1996,153:199-255
[5]Raper E S.Corrd.Chem.Rev.,1997,165:475-567 and references therein
[6]Xiong R G,Zhao H,You X Z,et al.Angew.Chem.Int.Ed.,2002,41:3800-3803
[7]Zhao H,Qu Z R,Xiong R G,et al.Chem.Soc.Rev.,2008,37:84-100
[8]Mura P,Olby B G,Robinson S D.Inorg.Chim.Acta,1985,97:45-52
[9]Tannai H,Tsuge K,Sasaki Y,et al.J.Chem.Soc.Dalton Trans.,2003:2353-2358
[10]Wilton-Ely J D E T,Schier A,Schmidbaur H.Organometallics,2001,20:1895-1897
[11]Wilton-Ely J D E T,Schier A,Mitzel N W,et al.Inorg.Chem.,2001,40:6266-6271
[12]Castano M V,Plasencia M M,Macias A,et al.J.Chem.Soc.,Dalton Trans.,1989,1:409-1411
[13]Ng V W L,Kuan S L,Weng Z Q,et al.J.Organomet.Chem.,2005,690:2323-2332
[14]Li Z H,Du S W,Wu X T.Polyhedron,2005,24:2988-2993
[15]Zhang X L,An N,Qiu Y E,et al.Acta Cryst.,2007,E63:m2432-m2433
[16]Tzeng B C,Wu Y L,Lee G H,et al.New J.Chem.(Nouv.J.Chim.),2007,31:199-201
[17]Tannai H,Tsuge K,Sasaki Y.Bull.Chem.Soc.Jpn.,2006,79:1223-1230
[18]Castano M V,Sanches A,Casas J S,et al.Inorg.Chim.Acta,1992,201:83-86
[19]Ma C,Li F,Jiang Q,et al.J.Organomet.Chem.,2004,689:96-104
[20]Sheldrick G M.SHELXS-97,Programm zur Lösung von Kristallstrukturen,Göttingen,1997.
[21]Antolini L,Cornia A,Fabretti A C,et al.J.Chem.Soc.,Perkin Trans.2,1993:417-420
Preparation and Crystal Structure of a Two-Dimensional Cadmium(Ⅱ)Coordination Polymer Containing Bismuthiol(Ⅱ)as a Bridging Ligand
WANG Wen-XiangZHAO Hong*
(Ordered Matter Science Research Center,School of Chemistry and Chemical Engineering,Southeast University,Nanjing 211189)
The hydrothermal reaction of CdCl2with 1,3,4-thiadiazole-2,5-dithiol affords one 2D network cadmium(Ⅱ)coordination polymer,[Cd(5-thioxo-4,5-dihydro-1,3,4-thiadiazole-2-thiolate)2]n.The structure was determined by single crystal X-ray diffraction,the crystal belongs to orthorhombic system with space group Pbcn,and a=1.2816(2)nm,b=1.1621(2)nm,c=0.78406(16)nm,V=1.1677(4)nm3,Z=4.Its fluorescence was measured.CCDC:689817.
cadmium complex;hydrothermal reaction;crystal structure;fluorescence
O614.24+2
A
1001-4861(2010)06-1109-04
2010-02-16。收修改稿日期:2010-04-01。
国家自然科学基金(No.20801012)和东南大学优秀青年教师项目(No.4007041027)资助。
*通讯联系人。E-mail:zhaohong@seu.edu.cn
王文祥,男,29岁,博士生;研究方向:金属配合物的铁电、介电和压电性质。

