乌司他定对急性坏死性胰腺炎大鼠合并肺损伤的影响
纪涛 汤志刚 邱陆军 黄强 李建生 许戈良
乌司他定对急性坏死性胰腺炎大鼠合并肺损伤的影响
纪涛 汤志刚 邱陆军 黄强 李建生 许戈良
目的探讨乌司他定(UTI)对急性坏死性胰腺炎大鼠(ANP)合并肺损伤时肺内ET-1和NF-κB表达及肺损伤的影响。方法60只SD大鼠按随机数字法分成假手术组、ANP组和UTI组,各20只。采用胆胰管逆行注射5%牛磺胆酸钠溶液1 ml/kg体重制备ANP模型,假手术组胰管注射等量生理盐水,UTI组在ANP制模成功后即从大鼠尾静脉注射UTI 10 000 U/kg体重。24 h后处死动物,测血清淀粉酶、TNF-α、肺组织湿/干重比,免疫组化法检测肺组织NF-κB和 ET-1蛋白表达以及使用TUNEL法检测细胞凋亡。结果UTI组术后24 h血清淀粉酶、TNF-α和肺湿/干重比分别为(5 648±378)U/L、(89.19±3.54)ng/L和4.55±0.07,较ANP组的(6 799±437)U/L、 (183.30±8.18) ng/L和4.89±0.20显著降低(Plt;0.05)。假手术组未见NF-κB和ET-1表达,未见凋亡细胞。UTI组NF-κB和ET-1阳性表达率分别为(19±3)%和(8±1)%,较ANP组的(25±2)%和(13±1)%显著降低(Plt;0.05)。UTI组细胞凋亡指数为13.75±1.25,较ANP组的6.90±0.85显著升高(Plt;0.05)。结论ANP时肺组织NF-κB和ET-1的高表达可能导致肺损伤。UTI能改善肺微循环,减轻肺炎症性损伤。
胰腺炎,急性坏死性; 急性肺损伤; 乌司他定
重症急性胰腺炎(SAP)病程进展凶险,易发生多器官功能障碍,其中肺脏最易受累。研究证实,炎症反应和微循环障碍参与SAP合并肺损伤的发生、发展[1]。本实验通过检测急性坏死性胰腺炎(ANP)大鼠肺组织ET-1和NF-κB的表达,探讨乌司他定(UTI)对ANP合并肺损伤的影响机制。
材料与方法
一、实验动物与分组
清洁级健康成年SD大鼠,体重250~300 g,雌雄不拘,由安徽医科大学实验动物中心提供。按随机数字法分为假手术(SO)组、ANP组和UTI组,每组20只。参考Ellison等[2]的方法,经胆胰管逆行注射5%牛磺胆酸钠1 ml/kg体重制备ANP模型。SO组胰管注射等量生理盐水。UTI组于ANP模型制作成功后从大鼠尾静脉注射UTI 10 000 U/kg体重。各组动物于模型建立后24 h处死。
二、观测指标及检测方法
1.血清淀粉酶、TNF-α检测: 血清淀粉酶采用BeckmanCX9 全自动生化分析仪检测,TNF-α采用ELASA法检测,严格按照试剂盒说明书操作。
2.肺组织湿/干比:开胸后立即取出肺右叶,用电子天平称湿重后置80℃烤箱烤24 h至恒重,计算肺湿/干重比值。
3.肺组织病理学检查:肺组织常规甲醛固定、石蜡包埋、切片、HE染色,光学显微镜下观察。
4.NF-κB和ET-1蛋白检测:采用免疫组化法。用PBS代替一抗作阴性对照,用已知阳性标本作阳性对照。胞质内出现棕褐色颗粒为阳性表达。计算5个高倍视野内阳性细胞占总细胞数的百分率。
5.细胞凋亡检测:采用原位末端标记TUNEL法。按说明书操作。显微镜下见细胞核中有棕褐颗粒,即为凋亡细胞。计算5个高倍视野100个胰腺细胞,以染色阳性细胞占总细胞数的百分率为凋亡指数(AI)。
三、统计学处理

结 果
一、血清淀粉酶、TNF-α含量的变化
SO组、ANP组和UTI组的血清淀粉酶含量分别为(862±43)U/L、(6 799±437)U/L和(5 648±378)U/L;TNF-α水平分别为(9.72±0.85)ng/L、(183.30±8.18)ng/L和(89.19±3.54)ng/L。ANP组和UTI组较SO组明显升高,UTI组又低于ANP组,差异具有统计学意义(Plt;0.05)。
二、肺组织湿/干重比值和病理改变
SO组、ANP组和UTI组的肺组织湿/干重比值分别为4.33±0.04、4.89±0.20和4.55±0.07。ANP组和UTI组较SO组明显升高,UTI组又低于ANP组,差异具有统计学意义(Plt;0.05)。
SO组肺组织未见明显异常;ANP组部分肺泡腔内有少量渗出合并出血,肺泡大小不一,有少许塌陷,肺泡壁明显增厚,毛细血管高度充血,胸腔内少量积液呈血性或乳糜性;UTI组肺泡壁轻度增厚,间质毛细血管充血、扩张,少量炎性细胞浸润,支气管管壁轻度水肿。
三、肺组织NF-κB和ET-1蛋白的表达
SO组肺组织无NF-κB和ET-1蛋白表达。ANP组和UTI组NF-κB和ET-1蛋白主要表达于肺泡及其周围的间质组织(图1)。ANP组和UTI组的NF-κB阳性表达率分别为(25±2)%和(19±3)%;ET-1阳性表达率分别为(13±1)%和(8±1)%。两组间差异均显著(Plt;0.05)。
四、肺组织细胞AI
SO组未见明显凋亡细胞,ANP组和UTI组凋亡细胞明显多于SO组(图1)。3组AI分别为0、6.90±0.85和13.75±1.25。UTI组的AI明显高于ANP组,差异有统计学意义(Plt;0.05)。
讨 论
SAP时各种激活的胰酶和过度激活的炎症介质释放入血,损伤胰腺外重要器官。由于肺的特殊解剖学结构,首当其冲成为胰酶和细胞因子损伤的重要器官[3]。因此调节和抑制酶及炎症介质的释放对治疗SAP合并肺损伤具有重要意义。
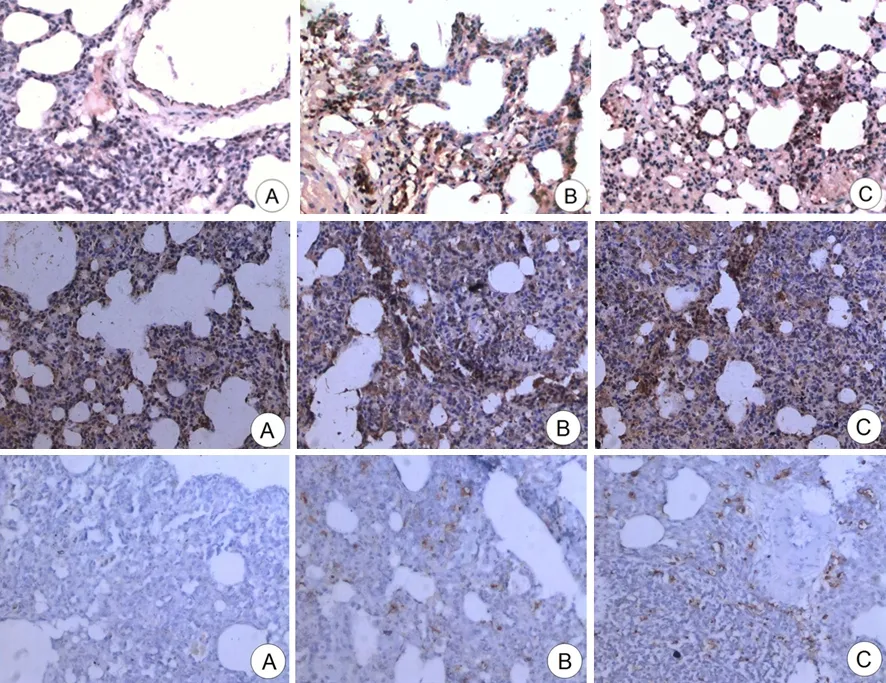
图1SO组(A)、ANP组(B)和UTI组(C)的肺组织NF-κB(上)、ET-1(中)表达和细胞凋亡(下)的变化(免疫组化、TUNEL ×100)
TNF-α在炎症反应中最早升高,并且处于始动地位,可以诱发中性粒细胞在肺组织内大量聚集,并释放炎性介质,引起肺损伤。NF-κB作为调控诸多炎性细胞因子的枢纽与其他转录因子共同作用参与炎症介质的诱导表达[4]。进一步研究发现,NF-κB的活化与细胞因子的表达及肺损伤之间呈正相关[5-6]。本实验动物在ANP制模后血淀粉酶、TNF-α水平及肺组织NF-κB表达、肺湿/干重比值均增加,表明ANP制模成功,肺组织受损害。
UTI是从人尿中提取制成的一种相对分子质量为67 000的糖蛋白,可抑制胰蛋白酶、胰淀粉酶、糜蛋白酶等活性,减轻各种胰液对胰腺组织自身的损伤。本实验给予UTI后TNF-α含量下降,肺组织NF-κB蛋白表达减少,肺湿/干重比值降低,肺组织炎症反应明显减轻,提示UTI可能通过抑制NF-κB活化、减少炎症介质释放而改善肺组织的损害。
ET-1是迄今为止发现的最强烈的血管收缩因子,ET与其受体结合将大大降低血流量,造成组织缺血、坏死、功能障碍甚至衰竭。Paulino等[7]报道,SAP患者血浆ET增高,本实验在ANP制模后,肺组织ET-1表达增加,结果一致。应用UTI处理后,肺组织ET-1表达较ANP组降低,肺湿/干重比值也低于ANP组,可见UTI的应用能明显改善肺组织的微循环,减少渗出,减轻ANP合并的肺损伤。
研究发现,凋亡参与了SAP发生发展的全过程[8]。由于凋亡过程中细胞膜保持完整,不伴有炎症介质和溶酶体释放,因此对机体具有保护效应。Koh等[9]报道,凋亡对急性肺损伤有明显的保护作用。本实验结果也显示,UTI组肺组织细胞的凋亡指数明显高于ANP组,表明UTI在抑制炎症反应的同时也能促进细胞凋亡。
[1] Surbatovic M,Jovanovic K,Radakovic S,et al.Pathophysiological aspects of severe acute pancreatitis-associated lung injury.Srp Arh Celok Lek,2005,133:76-81.
[2] Ellison EC,Pappas TN,Johnson JA,et al.Demonstration and characterization of the hemoconcentrating effect of ascitic fluid that accumulates during hemorrhagic pancreatitis.J Surg Res,1981,30:241-248.
[3] 黄晓丽,刘顺英,王国品.急性胰腺炎合并肺损伤的机制.胰腺病学,2007,7:64-66.
[4] Gulcubuk A,Altunatmaz K,Sonmez K,et al.Effects of curcumin on tumour necrosis factor-α and interleukin-6 in the late phase of experimental acute pancreatitis.J Vet Med A Physiol Pathol Clin Med,2006,53:49-54.
[5] Everhart MB,Han W,Sherrill TP,et al. Duration and intensity of NF-κB activity determine the severity of endotoxin-induced acute lung injury.J Immunol,2006,176:4995-5005.
[6] Abraham E,Nick JA,Azam T,et al.Peripheral blood neutrophil activation patterns are associated with pulmonary inflammatory responses to lipopolysaccharide in humans.J Immunol,2006,176:7753-7760.
[7] Paulino EC,de Souza LJ,Molan NA,et al.Neutrophils from acute pancreatitis patients cause more severe in vitro endothelial damage compared with neutrophils from healthy donors and are differently regulated by endothelins.Pancreas,2007,35:37-41.
[8] Yasuda T,Takeyama Y,Ueda T,et al.Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis.J Surg Res,2006,135:18-26.
[9] Koh H,Tasaka S,Hasegawa N,et al.Protective role of vascular endothelial growth factor in endotoxin-induced acute lung injury in mice.Respir Res,2007,25:60-73.
2008-08-04)
(本文编辑:吕芳萍)
Influenceofulinastatinonratswithacutenecrotizingpancreatitisassociatedlunginjury
JITao,TANGZhi-gang,QIULu-jun,HUANGQiang,LIJian-sheng,XUGe-liang.
DepartmentofGeneralSurgery,ProvincialHospitalofAnhui,AnhuiMedicalUniversity,Hefei230001,China
TANGZhi-gang,Email:tzg7031@163.com
Pancreatitis, acute necrotizing; Acute lung injury; Ulinastatia
AbatractObjectiveTo investigate the effects of Ulinastatin (UTI) on the expression of NF-κB and ET-1 in rats with acute necrotizing pancreatitis (ANP)-associated lung injury and morphology of lung tissue.Methods60 Sprague-Dawley rats were randomly divided into 3 groups with 20 rats in each group, including sham operation(SO) group, ANP group and UTI group. ANP was induced by injection of 5% sodium taurocholate (1 ml/kg) into pancreatic duct; normal saline was injected for SO group with same amount. UTI was injected for UTI group with the amount of 10 000 U/L via tail vein after ANP induction. The rats were sacrificed 24 h later. The contents of serum amylase, TNF-α and wet/dry weight ratio of the lung were measured. The expression of NF-κB and ET-1 protein were detected by immunohistochemical method. The level of apoptosis was detected by TUNEL.ResultsThe contents of serum amylase, TNF-α, and wet/dry weight ratio of the lungin in UTI group at 24 hours were (5 648±378)IU/L, (89.19±3.54)ng/L and 4.55±0.07, respectively; which were significantly lower than the corresponding (6 799±437)IU/L, (183.30±8.18)ng/L and 4.89±0.20 in ANP group (Plt;0.05). There was no NF-κB and ET-1 expression, and no apoptosis was present in SO group. The positive rates of NF-κB and ET-1 in UTI group were (19±3)% and (8±1)%, respectively, the corresponding values in ANP group were (25±2)% and (13±1)%, respectively (Plt;0.05). The level of apoptotic index in UTI group was 13.75±1.25, which was higher than that (6.90±0.85) in ANP group (Plt;0.05).ConclusionsThe high expression of NF-κB and ET-1 in lung tissue may cause lung injury. UTI could ameliorate the microcirculation and lung injury caused by inflammation.
10.3760/cma.j.issn.1674-1935.2009.02.009
安徽省卫生厅基金(05A004)
230001 合肥,安徽医科大学附属省立医院普外科
共同第一作者:汤志刚
汤志刚,Email:tzg7031@163.com

