Kinetic Study of Esterification of Lactic Acid with Isobutanol and n-Butanol Catalyzed by Ion-exchange Resins*
Qu Yixin (屈一新), Peng Shaojun (彭少君), Wang Shui (王水), Zhang Zhiqiang (张志强) and Wang Jidong (王际东)**
Kinetic Study of Esterification of Lactic Acid with Isobutanol and-Butanol Catalyzed by Ion-exchange Resins*
Qu Yixin (屈一新), Peng Shaojun (彭少君), Wang Shui (王水), Zhang Zhiqiang (张志强) and Wang Jidong (王际东)**
Beijing Key Laboratory of Bioprocess, Department of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
The esterification reactions of lactic acid with isobutanol and-butanol have been studied in the presence of acid ion-exchange resin Weblyst D009. The influences of catalyst loading, stirrer speed, catalyst particle size, initial reactant molar ratio and temperature on the reaction rate have been examined. Experimental kinetic data were correlated by using the Pseudo-homogeneous, Langmuir-Hinshelwood and Eley-Rideal models. Nonideality of the liquid phase was taken into account by using activities instead of molar fractions. The activity coefficients were calculated according to the group contribution method UNIFAC. Provided that the nonideality of the liquid is taken into account, the esterification kinetics of lactic acid with isobutanol and-butanol catalyzed by the acid ion-exchange resin can be described using all three models with reasonable errors.
kinetics, esterification, lactic acid, isobutanol,-butanol, ion exchange resin
1 INTRODUCTION
Lactic acid is used in wide applications in food industry for preservation and flavoring purposes, as well as in pharmaceutical and cosmetic industries. It is also used as an important raw material for the manufacture of biodegradable polymers [1, 2]. Its esters including isobutyl lactate and-butyl lactate are used as flavors and solvents with excellent properties [3]. The esterification reactions of lactic acid with alcohols are not only used in the production of the corresponding esters but also in the purification process of lactic acid. Therefore, kinetic data concerning esterification of lactic acid are necessary and important for design of these industrial processes.
For the esterification of lactic acid with alcohols, both homogeneous and heterogeneous catalysts can be used. However, most of the recent studies use heterogeneous solid catalysts, like acid ion-exchange resins, which can avoid the drawbacks of homogeneous catalyst, such as equipment corrosion and side reactions [4-10]. Dassy. [11] studied the kinetics of liquid phase synthesis and hydrolysis of butyl lactate catalyzed by acid ion-exchange resin in dioxane and toluene in a batch reactor. They found that the reaction rate was first order with respect to the amount of catalyst and the concentration of acid without considering the nonideality of the liquid mixture. Kumar and Mahajani [12] described the esterification of lactic acid with-butanol by reactive distillation catalyzed by acid ion-exchange resin. They used pseudo-homogeneousmodel to simulate the kinetic data also without considering the nonideality of the liquid mixture. Sanz. [9] investigated the reaction of lactic acid with methanol using Amberlyst 15 as catalyst. Three models including the Pseudo-homogeneous (PH), the Langmuir-Hinshelwood (LH) and the Eley-Rideal (ER) were used to correlate the experimental data. The PH model was found to represent the experimental data fairly well. Zhang. [10] studied the esterification of lactic acid [80% (by mass)] with ethanol in the presence of five acid ion-exchange resins. The LH model based on the selective adsorption of water and ethanol on the catalyst was found to be a more appropriate model to describe the kinetic behavior of these systems.
In this work, the esterification reactions of lactic acid with isobutanol and-butanol catalyzed by acid ion-exchange resins, Weblyst D009 and Weblyst D80 have been investigated. To provide a general kinetic model, experimental data were regressed by using different models based on homogeneous and heterogeneous approaches. To take into the nonideality of the liquid mixture, activities of the species in liquid phase, estimated by the group contribution method UNIFAC, were used instead of molar fractions.
2 EXPERIMENTAL
2.1 Chemical reagents
Isobutanol [purity≥99.5% by mass)] and-butanol [purity≥99.7% (by mass)] were supplied by Hongyan Reagent Co., Tianjin. Lactic acid [purity≥85% (by mass)] was purchased from Xilong Chemical Co., Guangdong. All of them were used without further purification. Lactic acid has two functional groups, a carboxyl and a hydroxyl. When the esterification reaction is carried out in high concentration of lactic acid, polylactic acid will be formed due to self-esterification [13]. Therefore, in this study dilute aqueous solution of lactic acid [20% (by mass)] was used in order to avoid the formation of polymers. Dilute lactic acid was obtained by adding distilled water into the lactic acid [85% (by mass)]. After mixing, the solutions were heated at 353.15 K for 1 week to increase the rate of formation of various oligomers of lactic acid [14]. The amount of polymerized lactic acid in the dilute lactic acid was considered negligible after determined by back titration [14].
2.2 Catalysts
Acid ion-exchange resins used in this study were purchased from Xiqiao Organic Material Co., Beijing. The properties of the catalysts obtained from the company are in Table 1. Prior to using for esterification reactions, the catalysts were first washed with methanol, then with deionized water and finally dried at 373 K for 24 h to completely remove moisture.
2.3 Apparatus and procedure
Esterification reactions of lactic acid with isobutanol and with-butanol were carried out in a four-necked flask of 500 ml capacity fitted with a reflux condenser, a thermometer and a magnetic stirrer. The temperature was maintained within an accuracy of ±0.5 K by an electric-heated thermostatic oil bath. The aqueous lactic acid solution and the catalyst were first charged into the reactor and heated to the desired temperature. Then isobutanol (or-butanol) was fed into the reactor. This time was considered to be the starting point of the reaction. Samples were withdrawn at regular time intervals for analysis. The catalyst loading was expressed as the mass ratio of the dry catalyst to the pure lactic acid in this article.
2.4 Analysis
All samples were weighed accurately by using an electronic balance with an accuracy of 0.0001 g. The amount of lactic acid was determined by titration with a standard sodium hydroxide solution using phenolphthalein as an indicator. Parallel tests indicated that the average error of the titration method was less than 2%. The samples were also analyzed with a gas chromatograph (GC) equipped a SW-FFAP capillary column (30 m×0.32 mm×0.5 μm) and a flame ionization detector (FID). Nitrogen with a purity of 99.99% was used as the carrier gas. Temperature of the injector and the detector were kept at 473.15 K. Initial temperature of the column was 323.15 K. After 5 min, the column temperature was increased to 473.15 K with a rate of 15 K·min-1. Finally it was maintained at 473.15 K for 20 min. No significant differences were observed between the results obtained by GC and titration method.
3 KINETIC MODELING
Esterification reactions catalyzed by ion-exchange resins can be described using different kinetic models based on homogeneous and heterogeneous approaches. Although PH model does not take into account the adsorption effect of the species in the reactant medium, it has been successfully used in high polar reaction media [7, 9]. The LH and the ER models both include the adsorption effects of the species in the reactant medium. It is obvious that these two models are more complicated than the PH model. The basic assumption of LH model is that all reactants are adsorbed on the catalyst surface before chemical reactions occur. The ER model assumed that the reaction takes place between adsorbed and non-adsorbed reactants. A general kinetic expression for all the three models is written as
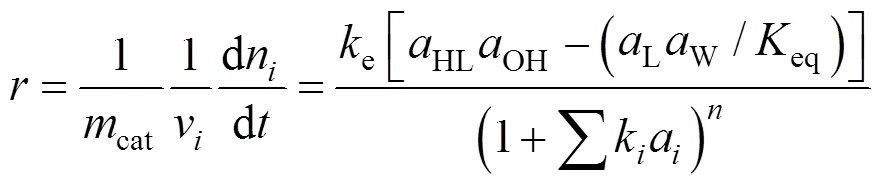
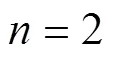
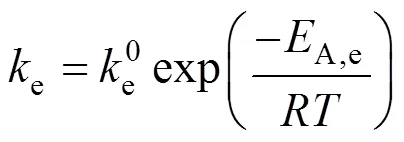
To consider the nonideality of the liquid phase, activities of components were used instead of molar fractions. The activity coefficients were calculated by using group contribution method UNIFAC [15-20]. The equilibrium constants for the two esterification reactions were calculated from the component concentrations at the equilibrium through Eq. (3):
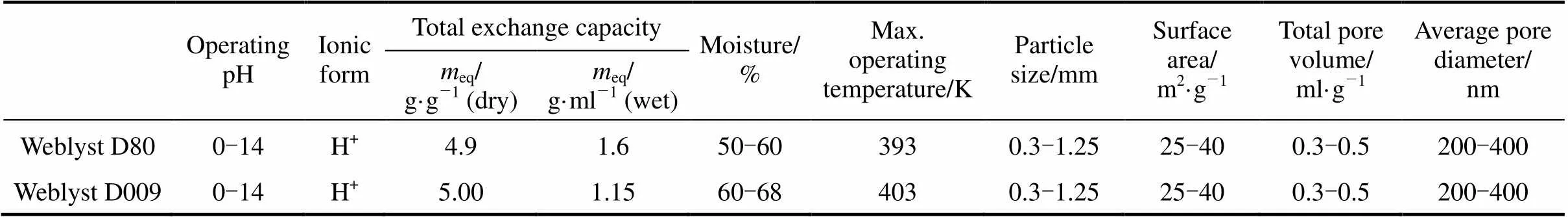
Table 1 Physical properties of the ion-exchange resins
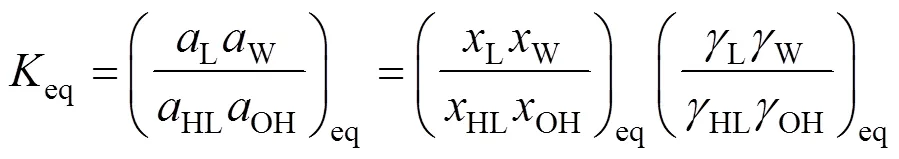
wherex,γare the molar fraction and activity coefficient of theth component, respectively, at equilibrium.
The kinetic parameters of the models were obtained by minimizing the sum of squared residuals between the experimental and calculated reaction rates as shown in Eq. (4) through the least square method:
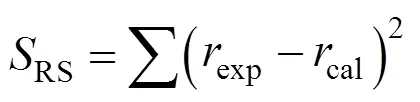
A fourth order Runge-Kutta method was used for integrating numerically the differential equations which describe the kinetic model with the previously determined parameters. The quality of the fit was estimated by the mean relative deviation of the molar fraction of lactic acid:
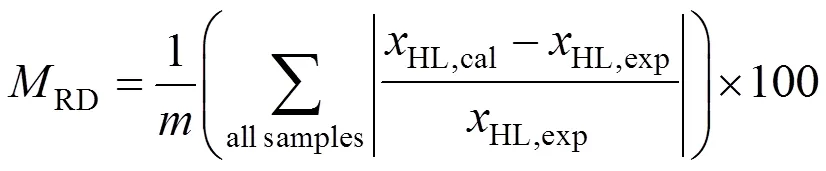
The subscripts “exp” and “cal” in Eqs. (4) and (5) are referring to the experimental and the calculated results andfor the total number of samples.
4 RESULTS AND DISCUSSION
4.1 Catalyst performance
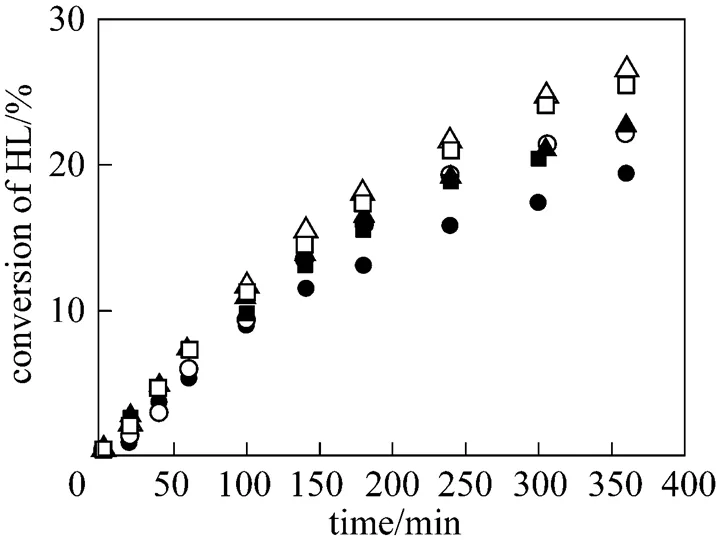
At the beginning, two acid ion-exchange resins, Weblyst D009 and Weblyst D80, were selected as the candidate catalysts. Their catalytic activities were first tested. Fig. 1 shows the comparison of the conversion of lactic acid obtained with Weblyst D009 and Weblyst D80 in the esterification reactions of lactic acid with isobutanol and-butanol. Results presented in Fig. 1 indicate that both Weblyst D009 and Weblyst D80 are effective catalysts.
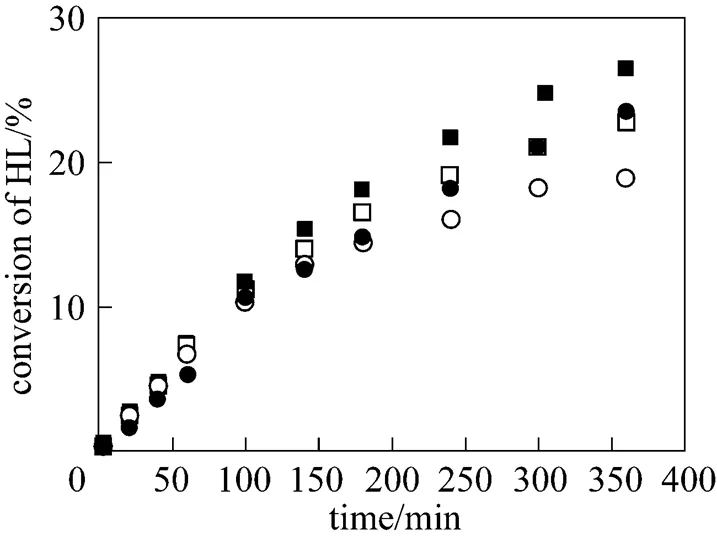
isobutanol:□ Weblyst D009;○ Weblyst D80
-butanol:■ Weblyst D009;● Weblyst D80
Although both catalysts have approximately the sameeqvalues, Weblyst D009 shows a noticeable higher activity than Weblyst D80 when the conversion of lactic acid is beyond 10%. During the experiments with Weblyst D80, it was noticed that in the reaction medium a larger part of Weblyst D80 cracked into small particles. This was not observed for Weblyst D009. The reasons for this difference are likely resulted from the differences in their compositions and/or structures. This means that besides the meq value, the composition and structure of an ion-exchange resin are also important factors in determining its catalytic effect. As a consequence, Weblyst D009 was chosen as the catalyst for further studies.
4.2 Elimination of mass transfer resistance
To evaluate the external mass-transfer resistance, the esterification reactions were carried out at different stirrer speeds of 200, 300, 400 r·min-1while keeping the other conditions unchanged. The results obtained were shown in Fig. 2. It can be seen that there was no significant difference in the conversion of lactic acid when the stirrer speeds are 300 and 400 r·min-1for both isobutanol and-butanol. This conclusion agrees with the previous works [21-23] which indicated that external diffusion does not control the overall reaction rate unless the stirrer speed is very low or the viscosity of reactant mixture is very high. To ensure the absence of external mass transfer resistance, the kinetic experiments in this work were all performed at a stirrer speed of 300 r·min-1.
isobutanol:● 200 r·min-1;■ 300 r·min-1;▲ 400 r·min-1
-butanol:○ 200 r·min-1;□ 300 r·min-1;△ 400 r·min-1
To evaluate the internal diffusion, the Weblyst D009 was screened into particles with three different size ranges, 0.8 mm-1.25 mm, 0.65 mm-0.8 mm, 0.3 mm-0.65 mm and esterification reactions with these three types of particles were carried out. No significant differences in the conversion of lactic acid were found for both esterification reactions with isobutanol and-butanol. This indicates that intra-particle diffusion resistance is negligible when the particle size is smaller than 1.25 mm for the esterification under the present reaction conditions. This is consistent with the previous studies [24, 25], which indicate that intraparticle diffusion resistances of the reactant in the ion exchange resin are not important.
4.3 Effect of temperature
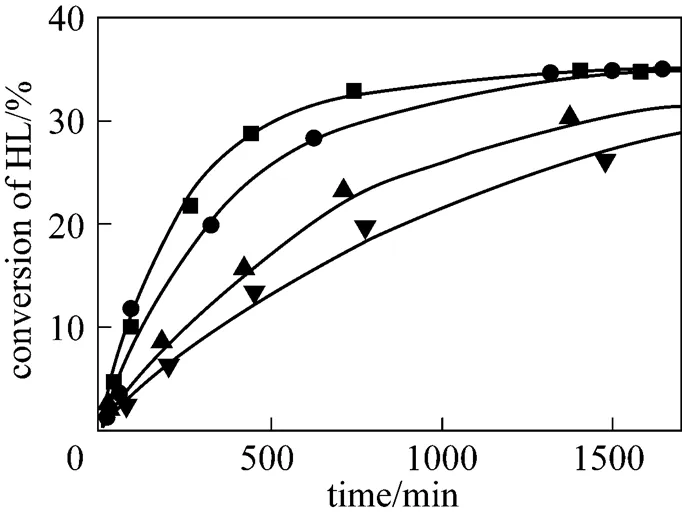
In order to investigate the effect of temperature, both esterification reactions were carried out in the temperature range from 333.15 to 363.15 K. Typical results are shown in Figs. 3 and 4. It can be seen that the reaction rate increases substantially with the increasing temperature. However, the equilibrium conversion was nearly equal in the range of temperatures studied in this work. Esterification reactions of acids with alcohols occur generally with small absolute values of reaction enthalpies, so the equilibrium conversion of lactic acid is a week function of reaction temperature. The similar effect of reaction temperature on the equilibrium conversion of lactic acid has been observed by Delgado. [26] in the reaction of lactic acid with ethanol and Sanz. [9] in the reaction of lactic acid with methanol.
▼ 333.15 K;▲ 343.15 K;● 353.15 K;■ 363.15 K
▼ 333.15 K;▲ 343.15 K;■ 353.15 K;● 363.15 K
From the equilibrium concentration of the reaction products, the equilibrium constants of the reactions in this work can be calculated according to


4.4 Effect of catalyst loading
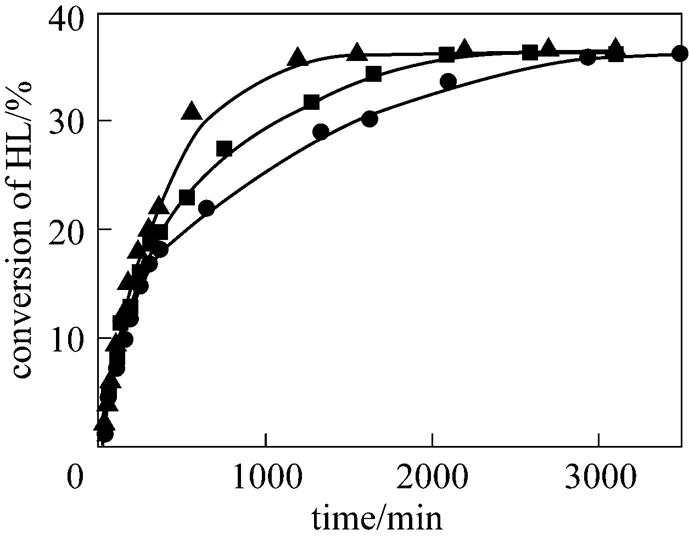
The catalyst mass loading was varied from 1.5% to 6% for the both esterification reactions. The conversions of lactic acid as a function of time with different catalyst loadings are shown in Fig. 6 for isobutanol at 353.15 K and Fig. 7 for-butanol at 363.15 K. As it can be seen from these figures, with increasing catalyst loading the conversion of lactic acid increases. A higher loading of catalyst results in reduction of the time required to reach the reaction equilibrium. No significant change in the equilibrium conversion of lacticacid is observed when the catalyst loading is changed.

Table 2 List of parameters of Eq. (1)
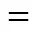
■-butanol;□ isobutanol
catalyst mass loading: ▲ 6%;■ 3%;● 1.5%
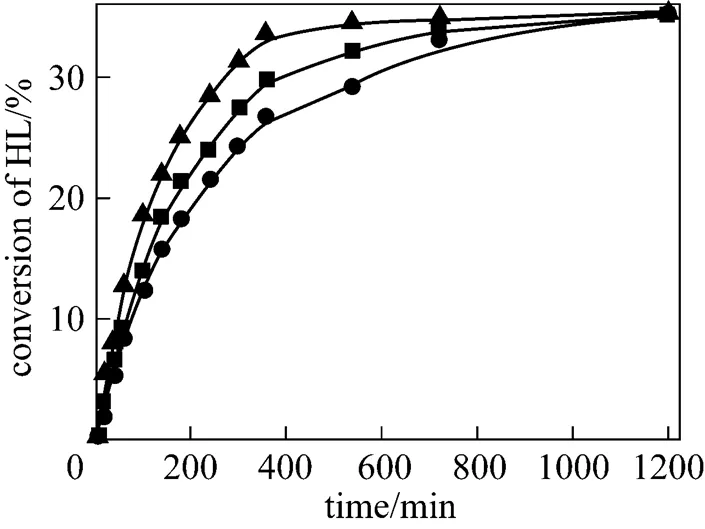
catalyst mass loading:▲ 6%;■ 3%;● 1.5%
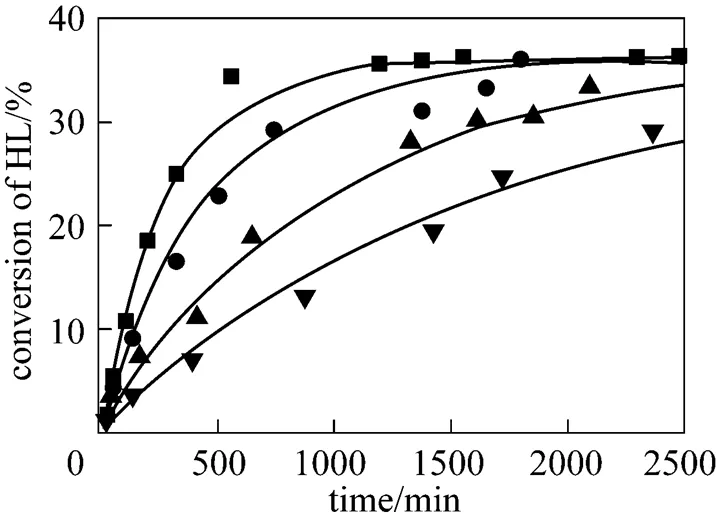
4.5 Effect of initial reactant molar ratio
To investigate the effect of the initial molar ratio of alcohols to lactic acid on the conversion of lactic acid, the initial molar ratio was changed from 1 to 4 for isobutanol (ISOH/HL) and 1 to 3 for-butanol (BUOH/HL). The results are given in Figs. 8 and 9. It can be seen that the conversion of lactic acid increases with increasing the initial molar ratio of alcohols to lactic acid. For the esterification with isobutanol, the equilibrium conversion of lactic acid increases from 15% to 40% when the initial molar ratioISOH/HLis raised from 1 to 4. For the esterification with-butanol, the equilibrium conversion of lactic acid increases from 15% to 35% when the initial molar ratioBUOH/HLis raised from 1 to 3. The results observed for the effect of the initial molar ratio of alcohols to lactic acid indicated that the equilibrium conversion of lactic acid can be effectively enhanced by using a large excess of alcohols, which is consistent with the results of Delgado. [26] who studied the esterification reaction of lactic acid with ethanol.
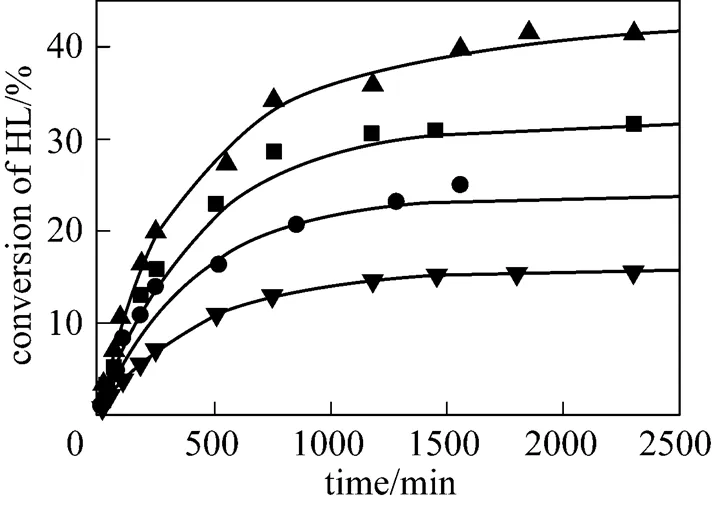
initial reactant molar ratioISOH/HL:▲ 4︰1; ■ 3︰1;● 2︰1;▼ 1︰1
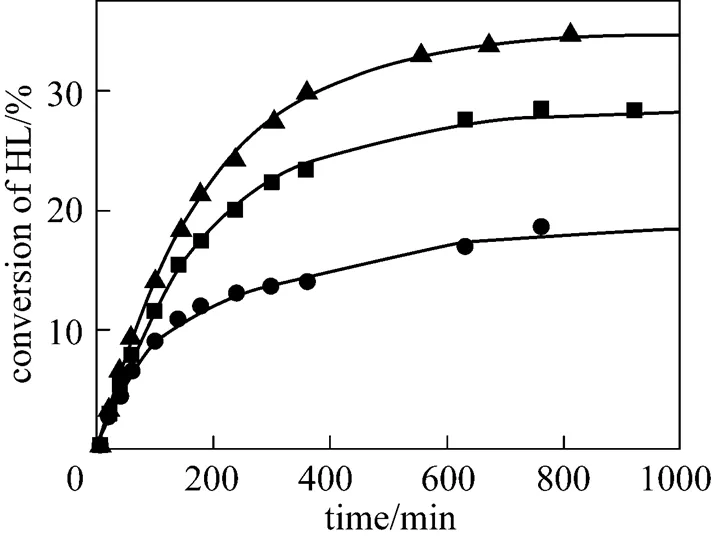
initial reactant molar ratioBUOH/HL:▲ 3︰1; ■ 2︰1;● 1︰1
4.6 Kinetic models
From the comparison of the values ofRSandRD, it is noticed that the assumption of ideal behavior of the liquid phase results in large errors. This is expected since the reaction systems are high polarity medium. When the nonideality of the liquid phase is taken into account, the PH model gives comparable accuracy as the ER and the LH model. The ER model and LH model give very close values ofRSandRD, implying that the assumptions made for the two models are reasonable. Provided that the nonideality of liquid phase is taken into account, it is difficult to discriminate one model from the others solely based on the values ofRSandRDsince the differences between the values ofRSand ofRDfor different models are very small.
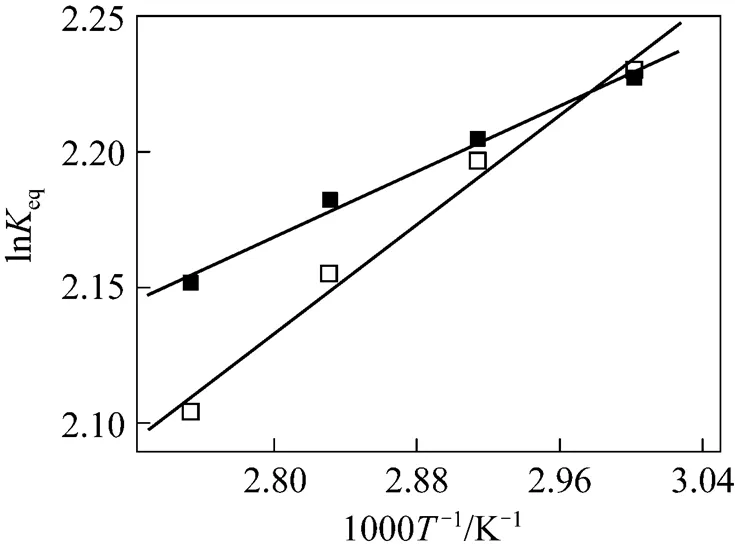
The Arrhenius plots for the esterification of lactic acid with isobutanol and with-butanol are given in Fig. 10. The continuous line represents the results obtained with LH model for isobutanol and the dash line represents the results obtained with the PH model for-butanol. A good linear relation between lneand 1/is observed.
然而早上7点,玛丽的父母却发现孩子倒在卫生间里,已经停止了呼吸。医生对悲伤的父母说,玛丽可能是倒下时头部受到撞击,导致猝死。
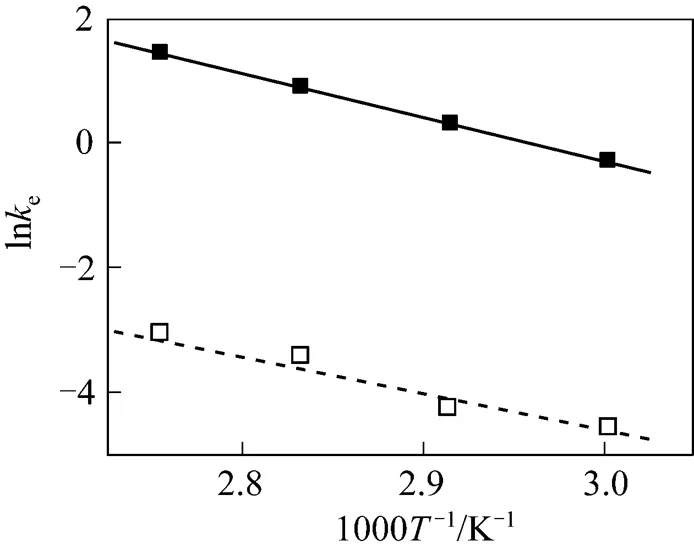
Figure 10 Arrhenius plot for the esterification of lactic acid with isobutanol and with-butanol (The continuous line represents the LH model, the dash line for the PH model.)
■ isobutanol;□-butanol
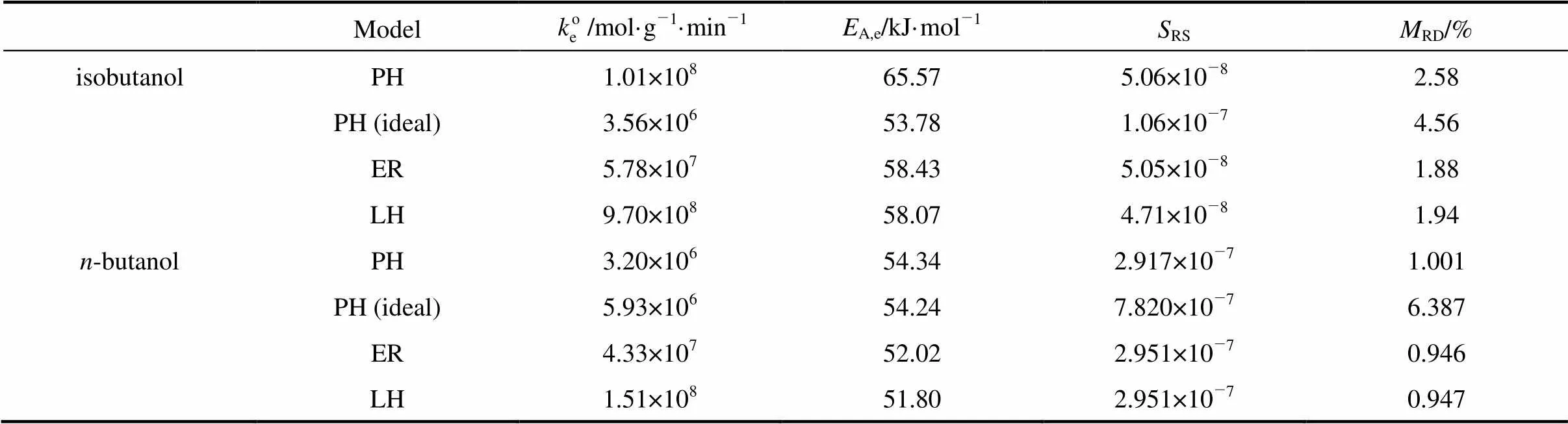
Table 3 Parameters of the kinetic models for the ion-exchange catalyzed esterification of lactic acid with isobutanol and n-butanol

Table 4 Comparison of the activation energies for the esterification of lactic acid with isobutanol and n-butanol
A comparison of the activation energies obtained in this work with those found in references is given in Table 4. For the esterification of lactic acid with isobutanol catalyzed by ion-exchange resin, no value of the activation energy was found in the literature. The activation energy found in this work is about 58 kJ·mol-1for the ER and the LH model and 65.6 kJ·mol-1for the PH model. For the esterification of lactic acid with butanol, the activation energy found in this work is about 52 kJ·mol-1for the ER and the LH model and 54.3 kJ·mol-1for the PH model. These valuesare 4-6 kJ·mol-1higher than that of Dassy. [11]who used Amberlyst 15 as the catalyst and dioxane and toluene as the solvents.
As compared to the previous work [11], the esterification of lactic acid with- and-butanol in aqueous solution was investigated using an acid ion-exchange resin Weblyst D009. Three kinetic models that take into account of the nonideality of the reaction medium were used to simulate the experimental data. When the nonideality of the reaction medium was considered, more accurate modeling results were obtained. Although all the three models that include the nonideality of the reaction medium give practically the same accuracy, it is prefer to use the PH model since this model has a simple form and is easy to use.
5 CONCLUSIONS
In this study, the kinetics of the esterification of lactic acid with isobutanol and-butanol catalyzed by acid ion-exchange resin Weblyst D009 was experimentally investigated. The reaction rate was found to increase with increasing reaction temperature and catalyst loading. The equilibrium conversion of lactic acid increased with increasing the molar ratio of the alcohols to lactic acid. The dependence of the equilibrium constant on the reaction temperature was in line with van’t Hoff equation. Three models, PH, LH and ER have been used to simulate the experimental data. To correct the nonideality of the liquid phase, activities by the group contribution method UNIFAC instead of molar fractions were used during the simulations. It was found that all these models were able to describe the kinetics of the esterification of lactic acid with butanol and isobutanol without high errors and gave comparable accuracy when the nonideality of the liquid phase was taken into account. The PH model is preferred due to its simple mathematical form.
NOMENCLATURE
activity
A,eapparent activation energy, kJ·mol-1
eqequilibrium constant
adsorption coefficient
eforward reaction rate constant, mol·g-1·min-1
RDmean relative deviation
number of experimental data
catmass of catalyst, g
nmole of component
gas constant , kJ·mol-1·K-1
reaction rate, mol·(g cat)-1·min-1
RSminimum sum
absolute temperature, K
time, min
molar fraction
activity coefficient
stoichiometric coefficient
Subscripts
BL butyl lactate
BUOH-butanol
calc calculated values
e esterification reaction
eq equilibrium
exp experimental values
HL lactic acid
IL isobutyl lactate
ISOH isobutanol
,components
L isobutyl lactate or butyl lactate
OH isobutanol or butanol
W water
1 Datta, R., Tsai, S.P., Bonsignore, P., Moon, S.H., Frank, J.R., “Technological and economic potential of poly (lactic acid) and lactic acid derivatives”,.., 16, 221-231 (1995).
2 Karin, H., Bflrbel, H., “Factors affecting the fermentative lactic acid production from renewable resources”,.., 26, 87-107 (2000).
3 Light Industry Research Institute in Jinan, Manual on Synthesis of Flavor, Light Industry Pub. Co., Beijing (1985). (in Chinese)
4 Yadav, G.D., Thathagar, M.B., “Esterification of maleic acid with ethanol over cation-exchange resin catalysts”,..., 52, 99-110 (2002).
5 Yadav, G.D., Thorat, T.S., “Kinetics of alkylation of-cresol with isobutylene catalyzed by sulfated zirconia”,...., 35, 721-732 (1996).
6 Peters, T.A., Benes, N.E., Holmen, A., Keurentjes, J.T.F., “Comparison of commercial solid acid catalysts for the esterification of acetic acid with butanol”,..., 297, 182-188 (2006).
7 Sanz, M.T., Murga, R., Beltran, S., Cabezas, J.L., Coca, J., “Autocatalyzed and ion-exchange-resin-catalyzed esterification kinetics of lactic acid with methanol”,...., 41, 512-517 (2002).
8 Troupe, R.A., Dimilla, E., “Kinetics of the ethyl alcohol-lactic acid reaction”,..., 49, 847-855 (1957).
9 Sanz, M.T., Murga, R., Beltran, S., Cabezas, J.L., Coca, J., “Kinetic study for the reactive system of lactic acid esterification with methanol: Methyl lactate hydrolysis reaction”,...., 43, 2049-2053 (2004).
10 Zhang, Y., Ma, L., Yang, J., “Kinetics of esterification of lactic acid with ethanol catalyzed by cation-exchange resins”,..., 61, 101-114 (2004).
11 Dassy, S., Wiame, H., Thyrion, F.C., “Kinetics of the liquid phase synthesis and hydrolysis of butyl lactate catalysed by cation-exchange resin”,...., 59, 149-156 (1994).
12 Kumar, R., Mahajani, S.M., “Esterification of lactic acid with-butanol by reactive distillation”,...., 46, 6873-6882 (2007).
13 Holten, C.H., Lactic Acid: Properties and Chemistry of Lactic Acid and Derivatives, Verlag Chemie, Copenhagen, Copenhagen (1971).
14 Vu, D.T., Kolah, A.K., Asthana, N.S., “Oligomer distribution in concentrated lactic acid solutions”,., 236, 125-135 (2005).
15 Rehfinger, A., Hoffman, U., “Kinetics of methyl tertiary butyl ether liquid phase synthesis catalyzed by ion exchange resin (I) Intrinsic rate expression in liquid phase activities”,..., 45, 1605-1607 (1990).
16 Ladisch, M., Westgate, P., Hendrickson, R., Brewer, M., “Framework for correlating composition dependent equilibrium conversion in methyl-butyl ether formation by ion-exchange catalysts”,...., 34, 2811-2816 (1995).
17 Rihko, L.K., Krause, A.O., “Kinetics of heterogeneously catalyzed-amyl methyl ether reactions in the liquid phase”,...., 34, 1172-1180 (1995).
18 Rihko, L.K., Kiviranta-Pääkkönen, P.K., Krause, A.O., “Kinetic model for the etherification of isoamylenes with methanol”,...., 36, 614-621 (1997).
19 Lee, M.J., Wu, H.T., Lin, H.M., “Kinetics of catalytic esterification of acetic acid and amyl alcohol over Dowex”,...., 39, 4094-4099 (2000).
20 Reid, R.C., Prausnitz, J.M., Poling, B.E., The Properties of Gases and Liquids, McGraw-Hill, New York (1987).
21 Chakrabarti, A., Sharma, M.M., “Cation ion exchange resin as catalyst”,.., 20, 1-45 (1993).
22 Yu, W., Hidajat, K., Ray, A.K., “Determination of adsorption and kinetic parameters for methyl acetate esterification and hydrolysis reaction catalyzed by Amberlyst 15”,..., 260, 191-205 (2004).
23 Darge, O., Thyrion, F.C., “Kinetics of the liquid phase esterification of acrylic acid with butanol catalysed by cation exchange resin”,...., 58, 351-355 (1993).
24 Seo, Y., Hong, W.H., “Kinetics of esterification of lactic acid with methanol in the presence of cation exchange resin using a pseudo-homogeneous model”,..., 33, 128-133 (2000).
25 Teo, H.T.R., Saha, B., “Heterogeneous catalysed esterification of acetic acid with isoamyl alcohol: Kinetic studies”,.., 228, 174-182 (2004).
26 Delgado, P., Sanz, M.T., Beltran, S., “Kinetic study for esterification of lactic acid with ethanol and hydrolysis of ethyl lactate using an ion-exchange resin catalyst”,..., 126, 111-118 (2007).
2009-03-19,
2009-07-12.
the National Basic Research Program of China (2007CB714300).
** To whom correspondence should be addressed. E-mail: jidongwang1963@yahoo.com.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Relative Humidity on Catalytic Combustion of Toluene over Copper Based Catalysts with Different Supports*
- Prediction of Critical Endpoints Based on the PR Equation of State*
- Kinetic Studies on Wheat Straw Hydrolysis to Levulinic Acid*
- Calculation of Transport Properties of CF4+Noble Gas Mixtures
- Synthesis of 4,4′-MDC in the Presence of Sulfonic Acid-functionalized Ionic Liquids*
- A New Study on Combustion Behavior of Pine Sawdust Characterized by the Weibull Distribution*
