Synthesis and Characterization of Novel Temperature and pH Responsive Hydroxylpropyl Cellulose-based Graft Copolymers
LI Xiaojun (李小军), YIN Minghui (尹明辉), ZHANG Guoliang (张国亮)* and ZHANG Fengbao (张凤宝)
Synthesis and Characterization of Novel Temperature and pH Responsive Hydroxylpropyl Cellulose-based Graft Copolymers
LI Xiaojun (李小军), YIN Minghui (尹明辉), ZHANG Guoliang (张国亮)* and ZHANG Fengbao (张凤宝)
School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
In this study, double-hydrophilic hydroxylpropyl cellulose (HPC) based copolymers with poly(N- isopropylacrylamide) (PNIPAM) and poly(acrylic acid) (PAA) as graft chains were synthesized and characterized. The release behavior of drug-loaded micelles was studied. The results show that the hydrophilicity of copolymers improves as the pH increases, whereas the hydrophobicity of copolymers enhances as the temperature increases, and all the phase behaviors are reversible. The diameter of micelles decreases and then increases with pH increase. It shows different micellizing behavior under acidic and basic conditions according to the temperature increase.release experiments, which used theophylline as a model drug, show that the micelles enhance pH sensitivity in the release process.
hydroxylpropyl cellulose, thermosensitive, pH sensitive, micelle, theophylline
1 INTRODUCTION
Polymeric micelles were first proposed as drug delivery systems in 1984 [1]. The study on drug carrier of polymeric micelles has been focused on block copolymers and graft copolymers with amphiphilic structure [2]. Hydrophilic blocks can be formed by various materials, including natural macromolecules like polysaccharide [3] and synthetic polymers like poly(ethylene glycol) (PEG) [4, 5]. Hydrophobic blocks vary in a wide chemical composition range, and the preferred hydrophobic blocks, just like poly(lactic acid) (PLA) [6, 7], should be biodegradable. The amphiphilic copolymers assemble to micelles in selective solvent-water system. Drugs, especially insoluble drugs, can be incorporated in the cores of micelles by physical entrapment. However, organic solvents, which are harmful to both human and environment, are inevitably involved in the preparation of copolymer micelles. Synthesis of double-hydrophilic copolymers was first reported in 1972 [8], but the significance of these copolymers was realized in recent years. These copolymers can form micelles in water without organic solvents by the stimuli-responsive of their chains, and they can change their solubility according to different conditions.
Hydroxylpropyl cellulose (HPC), an important soluble nonionic cellulose aether, is produced through the modification of hydroxyl group in the 2-, 3-, and 6-position of glucose ring unit [9, 10]. Hydroxylpropyl cellulose has many advantages such as biocompatibility and high-yield. Poly(N-isopropylacrylamide) (PNIPAM) is a thermosensitive polymer, which shows a certain lower critical solution temperature (LCST) in water at about 32°C. Recently, intensive studies have been focused on PNIPAM and its copolymers for its LCST near body temperature [11-14]. It is known that the phase transition and relative polymer conformation changes result from a delicate balance between the hydrophobic interaction and hydrogen bonding. Poly (acrylic acid) (PAA) presents hypercoiled conformation at low pH because of the hydrophobic interactions that are introduced by the polymer chains. However in basic condition, the carboxyl groups may ionize. Therefore, the increase in coulombic repulsive forces results in conformational transition from the hypercoiled to expanded form [13, 15], and the conformational change is reversible.
In this study, we investigated the micellization of the double-hydrophilic copolymers. For this purpose, the synthesis and characterization of HPC-based copolymers with PNIPAM and PAA as graft chains were reported. The phase transition and micellization of the double-hydrophilic copolymers were determined. In addition, we tried to examine the drug-loading ability and release behavior of the copolymer micelles.
2 EXPERIMENTAL
2.1 Materials
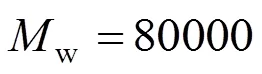
2.2 Synthesis of the graft copolymers [16]
Hydroxylpropyl cellulose (5 g) was dissolved in double-distilled water (100 ml) in a three-necked flask, which was kept in a water bath with magnetic stir at 25°C. The flask was purged with nitrogen for 30 min, and the nitrogen atmosphere was maintained throughout the polymerization. Certain volume of 0.1 mol·L-1ceric ammonium nitrate solution in 1.0 mol·L-1nitrate acid was added, stirred for 10 min, and then certain mass of NIPAM was added to the flask, and the reaction was continued for 4-5 h. The reaction was stopped by adding 1 mol·L-1sodium hydroxide solution. The reaction mixture was dialyzed in dialysis tube (cut-off 10000) for 3 d to get rid of PNIPAM homopolymers and other impurities. Then, the copolymer was reacted with certain volume of AA by the same method. The product was lyophilized to white floccule for at least 3 d.
2.3 Characterization of chemical structure and composition

1H-NMR spectra were obtained on NMR instrument (Varian,INVOA 500MHz) with D2O as solvent. The1H-NMR spectrum of HPC-g-PNIPAM showed the characteristic peaks of PNIPAM at 2.0 (CH2) and 1.5 (CH) compared with the spectrum of HPC. Besides these peaks, the1H-NMR spectrum of HPC-g- (PNIPAM&PAA) showed a proton peak at 10.1 (COOH).
The mass ratios of the original reactants (HPC︰ NIPAM︰AA) in the three reactions were 38.46︰38.46︰23.08, 45.46︰27.27︰27.27, and 45.46︰27.27︰27.27, and corresponding copolymers were named as HNAa1, HNAa2, and HNAb (synthesized by directly adding AA after 3 h) [17-19].The composition of copolymers were obtained by elementary analysis (Heraeus, Vanio-EL) and decomposition through cellulase [20]. The mass percent of PNIPAM was calculated by N mass fraction. Then, copolymers were dissolved in water with cellulase at pH 3.6 and 37°C for 3 d and precipitated in acetone, the lost mass was considered as the mass of HPC. The mass ratios (HPC︰PNIPAM︰PAA) in the resultant copolymers HNAa1, HNAa2, and HNAb were 50.16︰31.02︰18.82, 52.75︰25.68︰21.57, and 44.37︰22.86︰32.77, respectively.
2.4 Spectrophotometric phase-transition measurement
The LCST of the copolymers were determined by UV-Vis spectrometer (Unico, UV-2802H). The concentration of the polymeric solution used for the determination of phase transition was 0.5 mg·ml-1. We selected 480 nm as the analyzing wavelength. The sample solution was put in a sample holder with a temperature controlled circular system. Double-distilled water was adopted as reference for the measurement. The temperature range of the measurement was from 25 to 40°C. The temperature was increased to 0.5°C for every 10 min. The LCST were determined at the temperature of 50% decrease in optical transmittance.
2.5 Particle size distribution measurement
The particle size distribution of micelles was determined by dynamic light scattering (DLS, Brookhaven Instruments, BI-200SM). The concentration of the polymeric solution used for the determination was 1.0 mg·ml-1. The DLS experiments were performed with a He-Ne laser system, the fluctuations of scattering intensity at the scattering angle of 90° were recorded and transformed automatically. The measuring compartment was maintained at desired temperature with a temperature controlled system.
2.6 Characterization of micelle morphology
The morphology of micelles was characterized by scanning electron microscope (SEM, JEOL, JSM-6700F). The copolymer solution (1.0 mg·ml-1, 20 μl) was cast onto a fresh glass plate, and double- distilled water (40 μl) was added to prevent polymer forming membrane. The sample was lyophilized for 12 h and then coated with gold for observation.
2.7 Release of theophylline in vitro from the micelles
Drug-loaded micelles were prepared as follows: certain mass of theophylline and 100 mg copolymer were dissolved in 0.05 mol·L-1NaOH aqueous solution, and 0.05 mol·L-1HCl aqueous solution was added dropwise into the mixture with ultrasonic and magnetic stirring at 37°C. The mixture was placed in a water bath vibrator for 24 h at 37°C, and then the resulting solution was centrifugated for 30 min to get rid of unloaded drug. The suspended solution was lyophilized to drug loading micelles, and precipitate was used to calculate loading content.
The release experiments were performedas follows: theophylline or drug-loaded micelles (5 mg) were dissolved in buffer solution (10 ml) at various pH (3.6, 7.4, and 10.0). The solution was then poured in dialysis tube (cut-off 2000), and the dialysis tube was placed in a bottle containing 40 ml buffer solution as release medium. The release medium was stirred at 100 r·min-1and 37°C, the whole medium was removed and replaced by fresh buffer solution at desired times. The concentration of theophylline in the medium was determined by UV-Vis spectrometer at 271 nm.
3 RESULTS AND DISCUSSION
3.1 Phase transition behavior of graft copolymers
Figure 1 shows the curves of polymeric solution transmittance percentagetemperature at different pH. The LCST of HNAa1 and HNAa2 copolymers are slightly affected with pH change, whereas LCST of HNAb copolymer is significantly affected. This result is reasonable because PNIPAM and PAA segments are comparatively independent and hardly form random copolymer. When pH change, the protonation or ionization of carboxyl seldom affects PNIPAM chains, and the LCST of HNAa1, HNAa2 copolymers only transfer a little around 32°C.
Although HNAb was synthesized by directly adding AA during NIPAM grafted, PNIPAM and PAA inevitably form random copolymers, so the properties of them are affected by each other. Coulombic repulsive forces are weaken to improve interaction of hydrophobicity with pH decrease, whereas coulombic repulsive forces are strengthened to improve interaction of hydrophilicity with pH increase. As a result, LCST of HNAb copolymer falls to about 29°C in pH 3.6 solution and rises to about 34°C in pH 10.0 solution.

Figure 1 Curves of 0.5 mg·ml-1 polymeric aqueous solutiontransmittancetemperature at different pH
■ HNAal; ● HNAa2; ▲ HNAb
As shown in Fig. 1, the transition temperature range of the graft copolymers becomes narrower with the increase of PNIPAM content, and the transition point of the graft copolymers moves slightly to higher temperature when PNIPAM content becomes lower. The main reasons for this are as follows: first, the thermosensitivity of copolymers is mostly decided by PNIPAM chains, so the transition temperature range of the graft copolymers becomes smoother; the transition temperature of the graft copolymers moves slightly higher with PNIPAM content decrease. Second, the PNIPAM segments in the copolymers are separated and diluted by the PAA segments, and this polymer dilution effect is further strengthened with AA content increase. The polymer dilution effect inhibits the intrachain hydrophobic interaction of the thermosensitive segments. Besides, the coulombic repulsive forces among carboxyl anions become higher as the AA content increase, which also cause the intramolecular hydrophobic interaction to diminish.
3.2 Micellization of graft copolymers at different conditions
Figure 2 shows the mean diameter of HPC-g- (PNIPAM&PAA) copolymer micelles at different pH and temperatures determined by DLS. It is shown that the diameter of micelles decreases as the pH increases from 3.6 to 7.4, and then increases when pH increases from 7.4 to 10.0. The main reasons for that are as follows: the coulombic repulsive forces among carboxyl anions are weak, causing intense hydrophobicity of graft chains at low pH, which leads to vigorous aggregate of copolymers. Main chains need to be bended to satisfy aggregate requirement, but the chains of HPC are rigid to inhibit the bending behavior. As a result, the main chains cannot wrap branches to reduce the free energy of system, so plentiful macromolecules aggregate to form micelles, resulting in the increase of diameter. Although the hydrophobicity of graft chains slightly weakens in mid pH, a few macromolecules can wrap branches to form micelles. However, the hydrophobicity of graft chains further decreases at high pH to hardly cause the aggregation of copolymers, and the copolymers only form loose assemblies. Thus, micelles have big diameter.
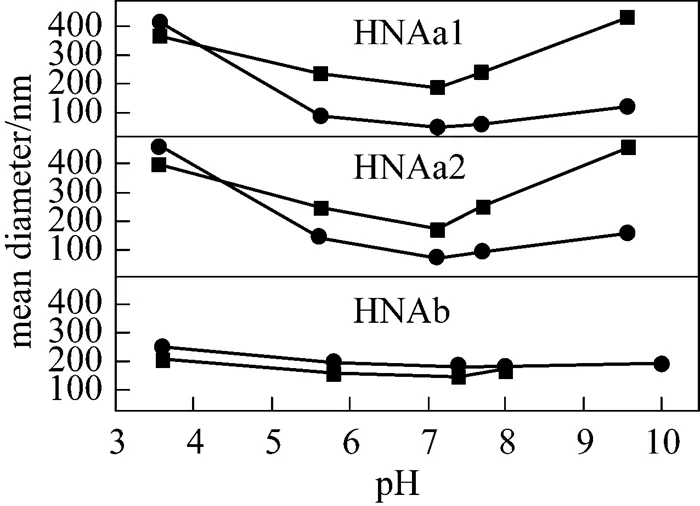
Figure 2 Mean diameter of HPC-g-(PNIPAM&PAA) copolymer micelles by DLS (Concentration is 1 mg·ml-1, HNAb cannot micellize at pH 10.0, 25°C)
■ 25°C;● 37°C
It can be observed from Fig. 2 that when temperature increases from 25 to 37°C, micelles diameter increases at low pH but decreases at mid and high pH. The reasons for that are as follows: when temperature changes, phase transition of PNIPAM chains improves the hydrophobicity of system. At low pH, fortified hydrophobicity causes more macromolecules aggregate to reduce the free energy of system, which causes the big diameter at 37°C, whereas in mid and high pH, fortified hydrophobicity offsets the deficiency of system hydrophobicity, the copolymer aggregations transit from loose assemblies to stable micelles, which causes the big diameter at 25°C.
The micellizing behavior of HNAb copolymer displays some characteristics for random copolymerization of NIPAM and AA. The diameter of HNAb micelles is obviously smaller than that of HNAa1 and HNAa2 at pH 3.6. The main reason is that dilution effect of PNIPAM and PAA chains decreases the hydrophobicity of copolymer to cause the abnormal phenomenon. Especially, HNAb cannot micellize at pH 10.0 and 25°C for intense hydrophilicity.
Figure 3, the SEM micrograph of HNAa1 micelles, shows the apparent morphology of micelles. The diameter is in line with the results from DLS. Further study is carried out to improve the sphericity and diameter distribution of the micelles.
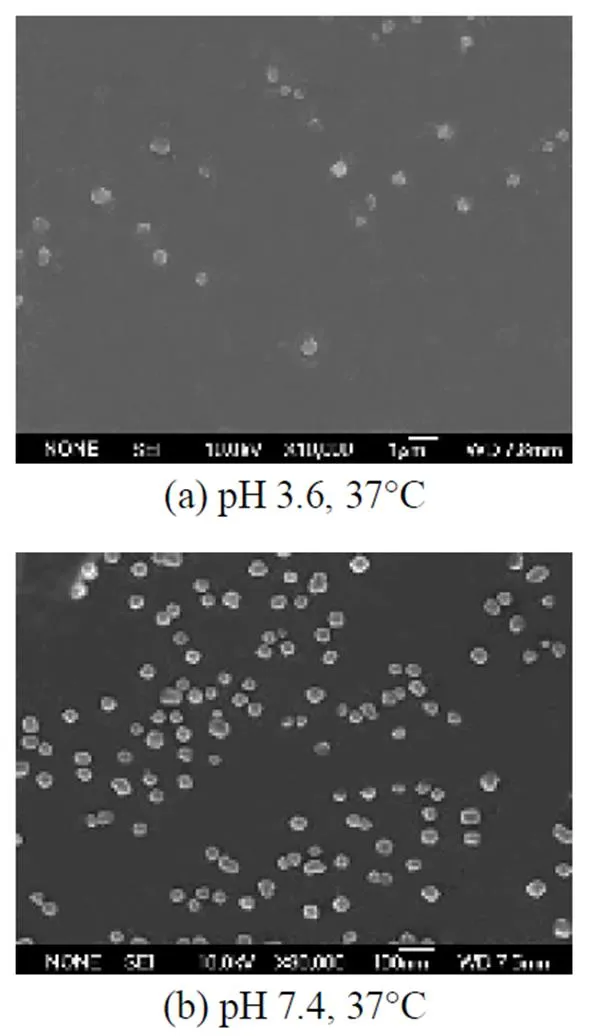
Figure 3 SEM micrograph of HNAa1 micelles
3.3 Theophylline loading and release
Theophylline was selected as a model drug because it has been widely used for bronchitis. The biological half-life of theophylline is brief and frequent doses may lead to unexpected side effects. In this study, theophylline was successfully loaded into copolymer micelles by physical entrapment. The loading of theophylline into HNAa1, HNAa2, and HNAb copolymers were 17.46%, 15.63%, and 12.17%, respectively. Obviously, loading content becomes higher as the PNIPAM content increases at 37°C because the hydrophobicity of cores is enhanced.
Figure 4 shows therelease behavior of series drug-loaded micelles. The results show that most of the theophylline releases in 1 h without micelles but drug-loaded micelles continually release in at least 10 h. Drug-loaded micelle release curves can be divided into three parts. Part I is the burst release phase; in this period, drug releases rapidly in about 2 h, and the content of drug released amounts to 50%-80% of total drug amount. Part II is the slow release phase; drug comes out from the interior of micelles with a relatively slow speed, this period releases 20%-30% of the total drug content in about 2 h. Part III is the equilibrium release phase; drug release content changes little and almost achieves an equilibrium state. From Fig. 4, it can be observed that the release effect of the three micelles becomes more satisfactory with PNIPAM content increase at 37°C. The results suggest that the appetency of micelle cores to theophylline strengthens as the hydrophobicity of copolymers increases, and free drug and adsorbed drug in micelle surface will drop off. So the burst release content decreases, and suspended release effect accordingly strengthens. Associated with loading content of micelles, the results are proven again.
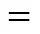
■ theophylline; ● HNAal; ▲ HNAa2; ◆ HNAb
To study the pH response of drug-loaded micelles, we determined the release behavior of HNAa1 micelles at different pH, the results are shown in Fig. 5. The suspended release of the three micelles became more satisfactory as the pH decreases. The results could be explained as follows: PAA chains mainly exist as carboxyl anion at high pH, and the coulombic repulsive forces cause structure of micelles loosening and swelling to release drug easily. Although it is difficult for PAA chains to ionize at low pH, the coulombic repulsive forces diminish to cause intense hydrophobicity of micelle cores. In this condition, the structure of micelles becomes compact to make against drug release. Otherwise, the solubility of theophylline increases in basic condition, which promotes drug diffusion into buffer solution. That is another possible reason for the results.

Figure 5 Comparison of theophylline release from HNAa1 micelles at different pH
pH: ■ 3.6; ● 7.4; ▲ 10.0
4 CONCLUSIONS
Double-hydrophilic copolymers with HPC as backbone and PNIPAM and PAA as graft chains were prepared by free-radical polymerization. This study shows that the hydrophilicity of copolymers improves as pH increases, whereas the hrdrophobicity of copolymers increases as the temperatures increases. All the phase behaviors are reversible. The diameter of micelles decreases and then increases as pH increases; it shows different micellizing behavior at acidic and basic conditions when temperature increases.
Therelease results, which used theophylline as model drug, show that the release behavior can be divided into burst release, the slow release and the equilibrium release periods. The micelles also enhance pH sensitivity in the release process.
1 Bader, H., Ringsdorf, H., Schmidt, B., “Watersoluble polymers in medicine”,..., 123-124, 457-485 (1984).
2 Jones, M.C., Leroux, J.C., “Polymeric micelles—A new generation of colloidal drug carriers”,...., 48 (2), 101-111 (1999).
3 Lemarchand, C., Gref, R., Couvreur, P., “Polysaccharide-decorated nanoparticles”,...., 58 (2), 327-341 (2004).
4 Topp, M.D.C., Dijkstra, P., Talsma, H., Feijen, J., “Thermosensitive micelle-forming block copolymers of poly (ethylene glycol) and poly(N-isopropylacrylamide)”,, 30 (26), 8518-8520 (1997).
5 Soliman, G.M., Winnik, F.M., “Enhancement of hydrophilic drug loading and release characteristics through micellization with new carboxymethyldextran-PEG block copolymers of tunable charge density”,..., 356 (1/2), 248-258 (2008).
6 Scholz, C., Iijima, M., Nagasaki, Y., Kataoka, K., “A novel reactive polymeric micelle with aldehyde groups on its surface”,, 28 (20), 7295-7297 (1995).
7 Kunii, R., Onishi, H., Machida, Y., “Preparation and antitumor characteristics of PLA/(PEG-PPG-PEG) nanoparticles loaded with camptothecin”,...., 67 (1), 9-17 (2007).
8 Kamachi, M., Kurihara, M., Stille, J.K., “Synthesis of block polymers for desalination membranes. Preparation of block copolymers of 2-vinylpyridine and methacrylic acid or acrylic acid”,, 5 (2), 161-167 (1972).
9 Ratajska, M., Boryniec, S., “Physical and chemical aspects of biodegradation of natural polymers”,..., 38 (1), 35-49 (1998).
10 Wang, C.Q., Tan, H.M., Dong, Y.P., Shao, Z.Q., “Trimethylsilyl hydroxypropyl cellulose: Preparation, properties and as precursors to graft copolymerization of [epsilon]-caprolactone”,..., 66 (10), 1165-1173 (2006).
11 Vesterinen, E., Dobrodumov, A., Tenhu, H., “Spin-labeled polyelectrolyte gels based on poly(N-isopropylacrylamide). Effects of the network structure and the gel collapse on the EPR spectra”,, 30 (5), 1311-1316 (1997).
12 Qiu, X., Wu, C., “Study of the core-shell nanoparticle formed through the ‘oil-to-globule’ transition of poly(N-isopropylacrylamide) grafted with poly(ethylene oxide)”,, 30 (25), 7921-7926 (1997).
13 Chen, G.H., Hoffman, A.S., “Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH”,, 373 (6509), 49-52 (1995).
14 Wei, H., Zhang, X.Z., Zhou, Y., Cheng, S.X., Zhuo, R.X., “Self- assembled thermoresponsive micelles of poly (N-isopropylacrylamide- b-methyl methacrylate)”,, 27 (9), 2028-2034 (2006).
15 Huang, M.F., Jin, X., Li, Y., Fang, Y.E., “Syntheses and characterization of novel pH-sensitive graft copolymers of maleoylchitosan and poly (acrylic acid)”,..., 66 (10), 1041-1046 (2006).
16 Athawale, V.D., Lele, V., “Syntheses and characterisation of graft copolymers of maize starch and methacrylonitrile”,.., 41 (4), 407-416 (2000).
17 Kim, S.Y., Cho, S.M., Lee, Y.M., Kim, S.J., “Thermo- and pH-responsive behaviors of graft copolymer and blend based on chitosan and N-isopropylacrylamide”,...., 78 (7), 1381-1391 (2000).
18 Kang, H.L., Liu, W.Y., He, B.Q., Shen, D.W., Ma, L., Huang, Y., “Synthesis of amphiphilic ethyl cellulose grafting poly(acrylic acid) copolymers and their self-assembly morphologies in water”,, 47 (23), 7927-7934 (2006).
19 Khan, A., “Preparation and characterization of N-isopropylacrylamide/ acrylic acid copolymer core-shell microgel particles”,.., 313 (2), 697-704 (2007).
20 Martinez-Richa, A., “Variation of intrinsic viscosity in the hydrolysis of hydroxyethylcellulose, and its relationship with resistance to enzymatic degradation”,, 39 (14), 3115-3118 (1998).
2008-03-13,
2008-09-21.
* To whom correspondence should be addressed. E-mail: zhangguoliang@tju.edu.cn
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- An Improved Fuzzy Predictive Control Algorithm and Its Application to an Industrial CSTR Process*
- Preparation, Characterization and Catalytic Behavior of 12-Molybdophosphoric Acid Encapsulated in the Supercage of Cs+-exchanged Y Zeolite*
- Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
- The Separation of Catechol from Carbofuran Phenol by Extractive Distillation*
- Electrochemical Performance of Nickel Hydroxide/Activated Carbon Supercapacitors Using a Modified Polyvinyl Alcohol Based Alkaline Polymer Electrolyte*
- Liquid Film Characteristics on Surface of Structured Packing*
