2,4-表油菜素内酯对干旱胁迫下西瓜幼苗生长及相关基因表达的影响
张伟 杨国慧 于辉
doi:10.6048/j.issn.1001-4330.2024.03.011
摘 要:【目的】研究外源2,4-表油菜素内酯(2,4-epibrassinolide,EBR)对干旱胁迫条件下西瓜幼苗生长以及相关基因表达的影响,为西瓜抗旱研究提供理论基础。
【方法】以西瓜品种齐红1号为材料,利用15%聚乙二醇(PEG6000)模拟干旱胁迫,设置3个处理CK(清水)、PEG和PEG+EBR,其中EBR的浓度为0.01 mg/L。检测不同处理后西瓜幼苗的生物量、光合作用指标、叶绿素含量、抗氧化酶活性以及相关基因的表达量。
【结果】喷施外源EBR可以缓解干旱胁迫对西瓜幼苗生长发育的抑制和损害程度,还可以通过增加应答基因的表达量,激活EBR信号转导途径,提高西瓜幼苗的耐旱性。喷施外源EBR西瓜幼苗株高、根长、地上鲜重、地下鲜重较PEG胁迫分别增加了2.41%、36.76%、1.88%和3.42%;而且也增强光合作用各项指标,抗氧化酶(POD和SOD)活性。油菜素内酯(BRs)信号转导途径关键应答基因BRI1、BIN2 、BES1 和DWF4的表达量也呈现不同程度变化,抗旱相关基因CDSP32和MYB101的表达量也有显著变化。
【结论】外源EBR能够有效缓解干旱胁迫对西瓜幼苗造成的损害,从而提高西瓜幼苗的抗旱性。
关键词:西瓜幼苗;2,4-表油菜素内酯;干旱胁迫;生长指标;基因表达
中图分类号:S182;S651 文献标志码:A 文章编号:1001-4330(2024)03-0615-08
收稿日期(Received):
2023-07-19
基金项目:
内蒙古自治区教育厅项目“鄂尔多斯地区西瓜自交系种质资源库和性状数据库的初步建立”(NJZY19376)
作者简介:
张伟(1984- ),男,内蒙古包头人,讲师,硕士,研究方向园艺植物遗传育种,(E-mail)weizhang880310@126.com
通信作者:
于輝(1976- ),男,黑龙江齐齐哈尔人,副教授,硕士,西瓜种植资源及育种,(E-mail)yh1166@sina.com
0 引 言
【研究意义】我国内蒙古光照充足,昼夜温差大,有利于西瓜营养物质的积累。但干旱已成为影响作物正常生长发育的危害因子之一[1]。西瓜生育期需水量大,苗期发育过程若受到干旱胁迫,将影响株高、鲜/干物质积累以及抗氧化酶活性[2]、氨基酸类代谢物含量[3],最终影响西瓜的产量和品质。因此需提高西瓜抗旱能力。油菜素内酯(Brassinosteroids,BRs)是20世纪70年代从甘蓝型油菜花粉中提取的一种植物生长素[4],其在植物生长发育、新陈代谢和逆境胁迫中起到关键作用[5-6]。研究外源2,4-表油菜素内酯(2,4-epibrassinolide,EBR)对干旱胁迫条件下西瓜幼苗生长以及相关基因表达的影响,对西瓜抗旱研究有重要意义。【前人研究进展】2,4-表油菜素内酯(EBR)和2,8-高油菜素内酯(HBR)是具有高活性的两种化合物[7],已应用于BR功能和相关转导研究。EBR存在于植物生长发育整个周期,主要作用是促进植物营养生长和生殖生长。EBR能提高毛竹实生苗光合能力从而促进其营养生长[8],也能通过促进羊草的株高、地上部分干重、纤维和蛋白的含量,从而增加羊草产量并提高品质[9]。EBR也能增强植物的抗逆性,喷施外源EBR 能够缓解烟草幼苗遭受干旱胁迫[10],可以提升辣椒的耐镉能力等[11]。外源EBR能够提高植物生理指标,如通过增强光合作用、增强抗氧化酶活性等有效缓解植物所受到的非生物胁迫[12-13]。冷害胁迫下西瓜幼苗经EBR处理后显著提高了保护物质的含量和抗氧化酶活性[14]。与EBR合成相关基因也有报道,如BRI1是油菜素内酯的受体蛋白,与植物株高和形态建成密切相关,水稻[15]、大麦[16]、玉米[17]、番茄[18]等作物BRI1基因的表达量均对植株株高的影响较大,其次是叶型等性状。植株受到非生物胁迫后,如低温[19]、高温[20]、盐胁迫[21]等,BRI1基因呈现上调趋势,从而协调植物内相关蛋白抵御逆境。BIN2 在BR信号传导过程中起到关键的作用,BRI1的下游调控蛋白,BRI1作为受体蛋白首先接收BR信号,再通过信号转导传递到BIN2等转录因子[22]。BES1是BR信号途中一个重要的转录因子,作用于细胞核内,正向调控BR的生物合成,从而促进植物生长以及防御反应[23-24]。外源EBR可以调节DWF4基因表达量下降,DWF4是维持BR平衡的关键基因[25]。CDSP32是一种类硫氧还蛋白,参与植物逆境调控途径,如干旱胁迫[26]、氧化胁迫[27]、镉胁迫[28]等,CDSP32蛋白均能正向应答非生物逆境。MYB101通过调节植物叶片形态来抵御干旱胁迫[29]。干旱胁迫会导致植物叶片发生卷曲, MYB101 基因在模式植物拟南芥中过量表达的会导致叶片向上卷曲。【本研究切入点】目前研究从生理生化和基因表达等方面揭示EBR可以有效调控植物的非逆境和逆境中的生长发育,但是关于EBR对干旱胁迫下西瓜幼苗的影响研究鲜见报道。需研究外源2,4-表油菜素内酯(2,4-epibrassinolide,EBR)对干旱胁迫条件下西瓜幼苗生长以及相关基因表达的影响。【拟解决的关键问题】利用聚乙二醇(PEG6000)模拟干旱胁迫,分析外源喷施10%浓度的EBR 对干旱胁迫后西瓜幼苗生长发育和生理特性的影响,为西瓜抗旱研究奠定理论基础。
1 材料与方法
1.1 材 料
供试西瓜品种为齐红1号,由东北农业大学园林园艺学院提供。
1.2 方 法
1.2.1 试验设计
选取籽粒饱满、大小一致的齐红1号种子约200粒,将其浸泡在1%的次氯酸钠溶液中消毒10 min,用灭菌蒸馏水冲洗3~4次,最后在滤纸上吸干多余的水分。将准备好的西瓜种子平铺在水培器中,置于发芽生长间25℃黑暗条件下催芽,待西瓜种子发芽后,挑选生长一致的置于网格盘进行水培,营养液为Hoagland,每2 d更换1次营养液,置于生长培养箱,16 h光照/8 h黑暗,温度为25℃/20℃,湿度为60%。待幼苗长至 2~4 片真叶时试验。设计3个处理(CK,PEG和PEG+EBR),每个处理选择生长一致的单株,首先将PEG+EBR组按文献的EBR浓度(0.01 mg/L)[30],于每天上午喷施1次EBR,CK组和PEG喷施清水,喷施方法为叶片双面均匀喷施直至形成均匀的水滴,连续喷施3 d,再进行干旱胁迫,采用聚乙二醇(PEG6000)15%(m/V)的比例与Hoagland营养液配置,将以上的PEG和PEG+EBR 2组干旱胁迫48 h。
1.2.2 测定及检测
(1)生物量:每个处理挑选9株幼苗,用清水冲洗根部,然后测量株高、根长,地上鲜重和地下鲜重,然后105℃杀青20 min,再在烘箱里60烘干至恒重,称量地上干重和地下干重,每个处理3次重复。
(2)光合指标:采用SPAD-502 Plus叶绿素仪检测每个处理后西瓜幼苗叶片的叶绿素相对含量。利用LI-6400便携式光合仪测定不同处理后西瓜幼苗叶片的光合作用指标。
(3)抗氧化酶活性:采用北京索莱宝科技有限公司出厂的超氧化物歧化酶(SOD) 活性和过氧化物酶(POD) 活性的测定试剂盒检测,每个处理测3次重复。
(4)RNA提取和qRT-PCR试验:筛选在拟南芥发表的与EBR抗旱相关基因,在西瓜数据库(http://cucurbitgenomics.org/organism/21)获得同源基因序列并设计引物,通过qRT-PCR检测基因表达量。表1
1.3 数据处理
数据利用Excle2010整理并分析。方差分析采用SAS V8,并利用Duncan 法在5%的水平上比较不同处理间差异的显著性。
2 结果与分析
2.1 EBR对干旱胁迫下西瓜幼苗生物量的影响
研究表明,PEG干旱胁迫后西瓜幼苗的株高、根长、地上鲜重、地下鲜重,地上干重、地下干重和根冠比均比CK显著降低,干旱胁迫对西瓜幼苗可以造成显著的伤害。喷施EBR后对西瓜幼苗的株高、根长比PEG干旱胁迫后有显著的增加,株高增加2.41%,根长增加36.76%。地上鲜重、地下鲜重、地上干重、地下干重和根冠比增加不显著,但是也有不同程度的增加,如地上鲜重增加1.88%,地下鲜重增加3.42%。喷施外源EBR可以通过促进根长的生长等,从而缓解干旱胁迫对西瓜幼苗造成的伤害。表2
2.2 EBR对干旱胁迫下西瓜幼苗光合作用指标的影响
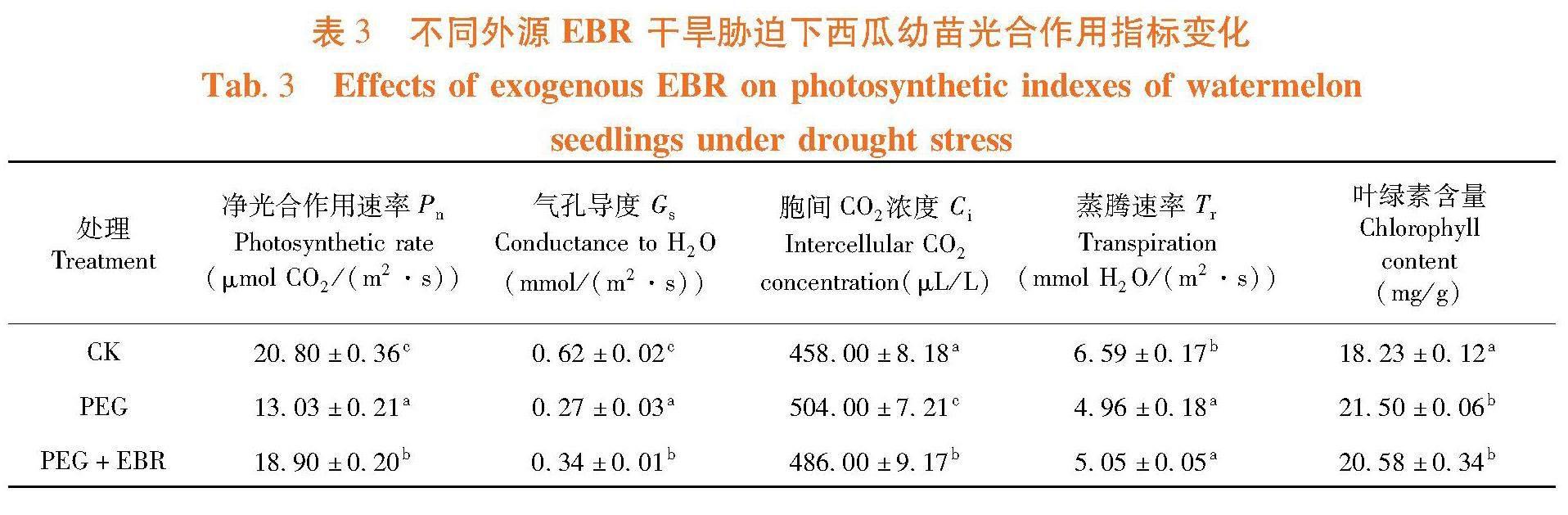
研究表明,西瓜幼苗的净光合作用速率降低37.34%,气孔导度降低56.44%,蒸腾速率降低24.70%,胞间CO2浓度提高10.04%,叶绿素含量提高17.89%。比PEG胁迫相比,喷施EBR后对西瓜幼苗的光合作用指标有明显增加,如净光合作用速率、气孔导度和蒸腾速率分别增加了31.04%、21.77%和1.95%,胞间CO2浓度和叶绿素含量也均显著降低,分别为3.70%和4.26%。噴施外源EBR可以显著缓解干旱胁迫对西瓜幼苗光合作用造成的影响。表3
2.3 EBR对干旱胁迫下西瓜幼苗抗氧化酶活性的影响
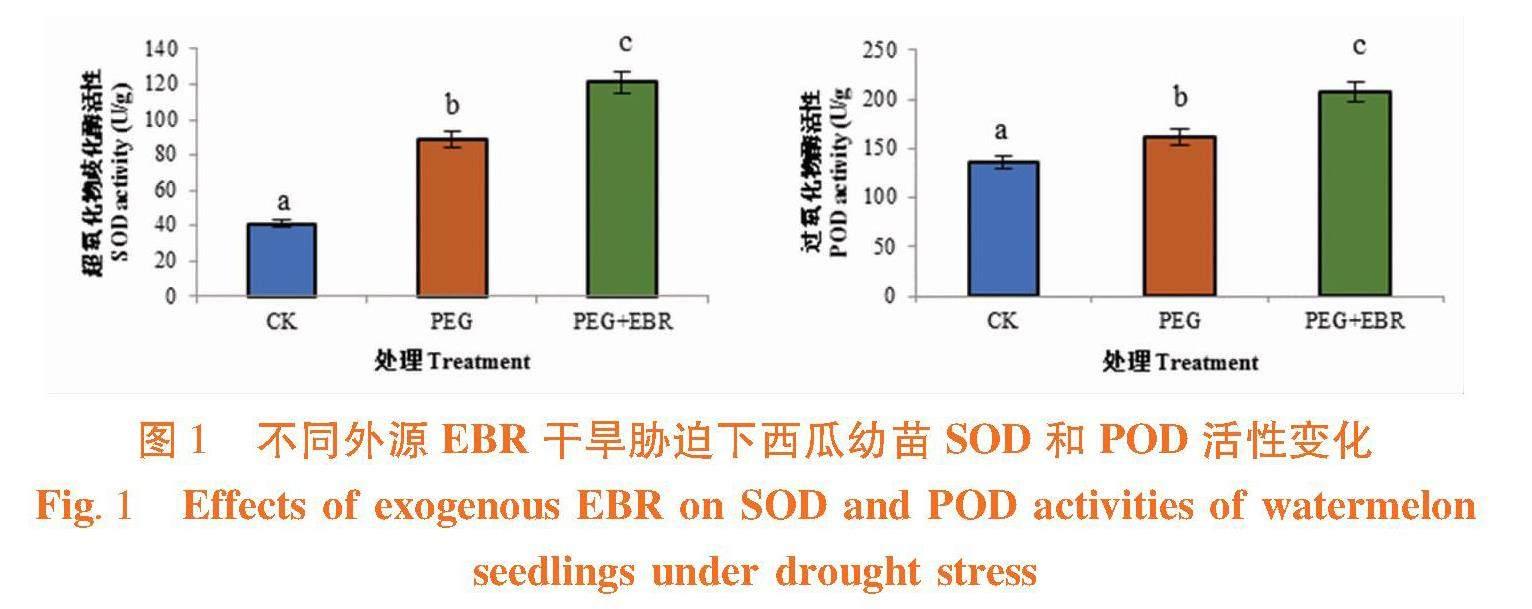
研究表明,PEG干旱胁迫西瓜幼苗后显著增加了超氧化物歧化酶(SOD)和过氧化物酶(POD)活性,与CK相比,分别增加18.56%和54.28%。与PEG干旱胁迫相比,喷施外源EBR后显著地增加了SOD和POD的活性,分别增加28.43%和38.16%。干旱胁迫下可以促进西瓜幼苗抗氧化酶的活性。图1
2.4 EBR对干旱胁迫下西瓜幼苗基因表达量的影响
研究表明,PEG干旱胁迫和PEG干旱胁迫后喷施外源EBR均对抗旱胁迫相关基因的表达量有一定影响。BRI1、BIN2 、BES1 和DWF4是与油菜素内酯合成相关基因,BRI1、BES1、和BES1的表达量与CK相比均有显著降低,而DWF4 表达量在西瓜幼苗受到干旱胁迫后与CK相比显著下降。然而当喷施外源EBR后,BRI1、BIN2、BES1 和DWF4基因的表达量与PEG干旱胁迫相比均有显著变化。当喷施外源EBR后,BRI1的表达量较PEG干旱胁迫增加5.2倍,BIN2的表达量增加2.4倍,BES1的表达量增加1.3倍,DWF4的表达量下降4.0倍。干旱胁迫西瓜幼苗可以抑制与油菜素内酯合成相关基因的表达,当喷施外源EBR可以促进油菜素内酯合成相关基因的表达。表1
PEG干旱胁迫后CDSP32的表达量与CK相比增加0.8倍,喷施外源EBR后较PEG干旱胁迫增加1.3倍。西瓜幼苗受到干旱胁迫后通过调节抗氧化酶相关基因的表达来抵御胁迫,外源EBR也可以增强抗氧化酶相关基因的表达。PEG干旱胁迫后MYB101的表达量与CK相比显著增加,但是喷施外源EBR后较PEG干旱胁迫显著降低1.4倍,外源EBR可以通过调节相关基因的表达改变西瓜幼苗的叶型卷曲程度。图2
3 讨 论
3.1
CDSP32主要作用于植物叶绿体结构[29-31]。MYB101通过调节植物叶片形态来抵御干旱[29],
干旱是影响西瓜品质和产量的主要环境因素之一[32]。干旱可降低西瓜植株的株高、鲜重和根冠比等[2],研究结果与以上结果一致,干旱胁迫会抑制西瓜幼苗的生长,不同部位反应不同,地上部分受害程度一般比地下部分更为严重。这样植物就会有效减少地上部分的水分蒸腾,保证根部的存活期限[33]。然而喷施EBR后能显著增加西瓜的株高、根长、地上和地下生物量,与丁丹阳等[10]研究喷施外源EBR可以显著提高烟草幼苗根系发育、生物量积累的结果一致。植物的光合作用也是感受干旱胁迫的一个重要指标,干旱胁迫后植物的光合速率普遍会下降,主要由于干旱胁迫下气孔感应后会应急关闭,从而尽量减少水分散失,由此可造成净光合速率下降、气孔导度下降以及蒸腾速率下降,最终形成胞间CO2浓度增加。研究西瓜幼苗在受到干旱胁迫后同样出现类似现象,但是当喷施外源EBR后,以上现象出现缓解状态,与赵小强等[34]和丁丹阳等[10]对玉米幼苗和烟草幼苗干旱胁迫后喷施EBR对光合作用各项指标显著提高的结果相似。EBR在增加植物地上部分的株高、调控植物根系发育方面具有决定性作用[35],干旱胁迫后外源喷施EBR能够增加植物净光合速率从而缓解逆境胁迫,但是作用机制尚未明确,有可能是EBR参与光合作用中的碳固定,从而克服气孔关闭的限制因素并提高光合作用[36]。
3.2
植物在受到逆境胁迫后产生大量的活性氧(Reactive Oxygen Species,ROS),而植物本身会有自我保护系统,清除多余的活性氧避免对植物造成损害,而这些清除剂大多数是抗氧化酶。研究西瓜幼苗受干旱胁迫后,SOD和POD的活性与CK相比显著增加,应急抵御水分缺失。当喷施EBR后,SOD和POD的活性急剧上升,EBR可以提高抗氧化酶的活性从而加速清除过量的ROS,达到缓解干旱胁迫的效果。以上结果与许金亮等[37]研究烟草对喷施EBR抵抗低温胁迫过程中,抗氧化酶活性显著增加一致。EBR对提高干旱胁迫下抗氧化酶活性来增强植物细胞膜的稳定性,最终提高植物抗旱能力[38]。
3.3
目前对BRs的信号通路的研究已经构建详细的信号传导模型[35]。研究选择在信号传导模型中关键的4个基因进行分析,其中BRI1是BRs的受体蛋白、BIN2 和BES1是通路中2个关键的调控因子,DWF4在调节BRs的平衡中起关键作用。研究结果与各个基因在信号通路中的作用关系一致,如BRI1、BIN2 和BES1基因的表达量在西瓜幼苗受到干旱胁迫后均呈现上升趋势,喷施EBR后基因的表达量显著增加,以上3个关键基因均在正向调控植物抵御逆境,与前人研究结果相似[39-41]。而DWF4基因的表达量呈现的是负向调控,与兰彩耘等[42] 研究结果一致。研究显示与抗旱相关的2个基因CDSP32和MYB101的表达量在西瓜幼苗受到干旱胁迫后也受到不同程度的影响,喷施外源EBR后两个基因的表达量也发生了一定变化。西瓜幼苗的抗旱过程也通过调控相关基因的表达,从而达到缓解干旱胁迫的影响。
4 结 论
西瓜幼苗干旱胁迫后其生长发育受到严重影响,而喷施外源EBR后可以通过增加西瓜幼苗的生物量、光合作用强度、抗氧化酶活性等缓解干旱胁迫造成的损害,从而提高西瓜幼苗的抗旱性。同时喷施外源EBR后,可以促进内源BR信号转导相关基因和抗旱相关基因的表达。喷施外源EBR西瓜幼苗株高、根长、地上鲜重、地下鲜重较PEG胁迫分别增加了2.41%、36.76%、1.88%和3.42%。
参考文献(References)
[1]
Chandra P,Wunnava A,Verma P,et al.Strategies to mitigate the adverse effect of drought stress on crop plants—influences of soil bacteria: a Review [J].Pedosphere, 2021,31(3): 496-509.
[2] 何亚萍,王春霞,闫星,等.9份西瓜种质苗期抗旱性鉴定[J].中国瓜菜,2020,33(12): 14-21.
HE Yaping,WANG Chunxia,YAN Xing,et al.Screening of drought resistance of nine watermelon germplasm at seedling stage[J].China Cucurbits and Vegetables,2020,33(12): 14-21.
[3] 賈斌,高龙飞,张卫华,等.西瓜苗期干旱胁迫下的代谢组学分析[J].分子植物育种,2023,21(21):7161-7170.
JIA Bing,GAO Longfei,ZHANG Weihua,et al.Metabolomics analysis of watermelon seedlings under drought stress [J].Molecular Plant Breeding,2023,21(21):7161-7170.
[4] Grove M D ,Spencer G F,Rohwedder W K,et al.Brassinolide,a plant growth-promoting steroid isolated from Brassica napus pollen[J].Nature,1979,281(5728): 216-217.
[5] Sreeramulu S,Mostizky Y,Sunitha S,et al.BSKs are partially redundant positive regulators of brassinosteroid signaling in Arabidopsis.[J].Plant Journal for Cell & Molecular Biology,2013,74(6): 905-919.
[6] Sharma I,Bhardwaj R,Pati P K.Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety pusa basmati-1[J].Journal of Plant Growth Regulation,2015,34(3): 509-518.
[7] Li J,Yang P,Kang J G,et al.Transcriptome analysis of pepper (capsicum annuum) revealed a role of 24-epibrassinolide in response to chilling [J].Frontiers in Plant Science,2016,29(7):1281.
[8] 李启程,余学军.外源油菜素内酯对毛竹实生苗生理特性的影响[J].浙江农林大学学报,2021,38(1): 120-127.
LI Qicheng,YU Xuejun.Effects of exogenous BR on physiological characteristics of phyllostachys edulis seedlings [J].Journal of Zhejiang A&F University,2021,38(1): 120-127.
[9] 胡勇军,韩德复,郭继勋.油菜素内酯对羊草人工草地产量及其品质的影响[J].长春师范学院学报,2007,26(4): 61-64.
HU Yongjun,HAN Defu,GUO Jixun.Effect of Brassinolide (BR) on the quality and the yield of leymus chinensis growing in the sown grassland [J].Journal of Changchun Normal University,2007,26(4): 61-64.
[10] 丁丹陽,张璐翔,朱智威,等.叶面喷施2,4-表油菜素内酯对烟草抗旱性的影响[J].中国烟草科学,2018,39(4): 50-57.
DING Danyang,ZHANG Luxiang,ZHU Zhiwei,et al.Effect of leaf spray 2,4-epibrassinolide on drought resistance of tobacco[J].Chinese Tobacco Science,2018,39(4): 50-57.
[11] 雷阳,乔宁,白扬,等.表油菜素内酯对重度镉胁迫下辣椒幼苗生理特性及抗逆基因的影响[J].华北农学报,2021,36(5): 99-106.
LEI Yang,QIAO Ning,BAI Yang,et al.Effects of Epibrassinolide on Physiological Characteristics and Resistance Genes of Pepper Seedlings under Severe Cadmium Stress [J].Acta Agriculturae Boreali-Sinica,2021,36(5): 99-106.
[12] Xia X J,Wang Y J,Zhou Y H,et al.Reactive Oxygen Species Are Involved in Brassinosteroid-induced Stress Tolerance in Cucumber [J].Plant Physiology,2009,150(2): 801-814.
[13] Bajguz A,Hayat S.Effects of brassinosteroids on the plant responses to environmental stresses [J].Plant Physiology and Biochemistry,2009,47(1): 1-8.
[14] 范小玉,张显.油菜素内酯对低温弱光胁迫下西瓜幼苗耐冷性的影响[J].北方园艺,2012,(7): 5-8.
FAN Xiaoyu,ZHANG Xian.The effect of brassinolide on chilling resistance of watermelon seedlings under low temperature and poor light stress [J]. Northern Horticulture,2012,(7): 5-8.
[15] Sakamoto T,Morinaka Y,Ohnishi T,et al.Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice [J].Nature Biotechnology,2006,24(1): 105-109.
[16] Chono M,Honda I,Zeniya H,et al.A semi dwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteriod receptor.[J].Plant Physiology,2003,133(3):1209-1219.
[17] Kir G,Ye H X,Nelissen H,et al.RNA interference knockdown of brassinosteroid insensitive1 in maize reveals novel functions for brassinosteroid signaling in controlling plant architecture [J].Plant Physiology, 2015,169(1):826-839.
[18] Montoya T,Nomura T,Farrar K,et al.Cloning the tomato Curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase Tbri1/sr160 in plant steroid hormone and peptide hormone signaling[J].Plant Cell,2002,14(12): 3163-3176.
[19] Kim S Y,Kim B H,Lim C J,et al.Constitutive activation of stress-inducible genes in a brassinosteroid-insensitive 1 (bri1) mutant results in higher tolerance to cold[J].Physiologia Plantarum,2010,138(2): 191-204.
[20] Martins S,Dohmann E M N,Cayrel A,et al.Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination [J].Nature Communications,2015,6: 6151.
[21] Goddard R,Peraldi A,Ridout C,et al.Enhanced disease resistance caused by bri1 mutation is conserved between brachypodium distachyon and barley (hordeum Vulgare).[J].Molecular Plant Microbe Interactions: MPMI,2014,27(10):1095-1106.
[22] 王斐,何偉,闫海芳.油菜素甾醇信号转导的调控机制[J].植物生理学报,2013,49(12): 1309-1318.
WANG Fei,HE Wei,YAN Haifang.Regulation mechanism of brassinosteroids signal transduction [J].Plant Physiology Journal, 2013,49(12): 1309-1318.
[23] Yang J N,Thames S,Best N B,et al.Brassinosteroids modulate meristem fate and differentiation of unique inflorescence morphology in setaria viridis [J].Plant Cell, 2018,30(1):48-66.
[24] Yin Y H,Wang Z Y,Mora-Garcia S,et al.BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation [J].Cell,2002,109(2):181-191.
[25] Banco瘙塂 S Nomura T,Sato T,et al.Regulation of transcript levels of the arabidopsis cytochrome p450 genes involved in brassinosteroid biosynthesis [J].Plant Physiology,2002,130(1): 504-513.
[26] Eymery F,Rey P.Immunocytolocalization of CDSP 32 and CDSP 34,two chloroplastic drought-induced stress proteins in solanum tuberosum plants [J].Plant Physiology and Biochemistry,1999,37(4): 305-312.
[27] Broin M,Cuiné S,Eymery F,et al.The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage [J].The Plant Cell,2002,14(6): 1417-1432.
[28] Zhang F G,Xiao X,Yan G X,et al.Association mapping of cadmium-tolerant QTLs in Brassica Napus L.and insight into their contributions to phytoremediation[J].Environmental and Experimental Botany,2018,155: 420-428.
[29] An R,Liu X Y,Wang R,et al.The over-expression of two transcription factors,ABS5/BHLH30 and ABS7/MYB101,leads to upwardly curly leaves [J].Plos One, 2014,9(9):e107637.
[30] Gong H J,Zhu X Y,Chen K M,et al.Silicon alleviates oxidative damage of wheat plants in pots under drought [J].Plant Science,2005,169(2):313-321.
[31] Broin M,Rey P.Potato plants lacking the CDSP32 plastidic thioredoxin exhibit overoxidation of the BAS1 2-cysteine peroxiredoxin and increased lipid peroxidation in thylakoids under photooxidative stress [J].Plant Physiology,2003,132(3):1335-1343.
[32] Guo S G,Shu H G,Zhang H Y,et al.Comparative transcriptome analysis of cultivated and wild watermelon during fruit development [J].Plos One,2015,10(6):e0130267.
[33] 樊正球.干旱環境胁迫下的植物分子适应机理及其应用研究[D].上海:复旦大学,2004.
FAN Zhengqiu.Study on plant molecular adaptation to drought stress and its application [D].Shanghai:Fudan University,2004.
[34] 赵小强,任续伟,张金乾,等.外源2,4-表油菜素内酯对干旱胁迫下青贮玉米幼苗生长和光合特性的影响[J].分子植物育种,2023,21(10),3371-3382.
ZHAO Xiaoqiang,REN Xuwei,ZHANG Jinqian,et al.Effects of exogenous 2,4-epibrassinolide on growth and photosynthetic characteristics of Silage Maize Seedlings under drought stress[J].Molecular Plant Breeding,2023,21(10),3371-3382.
[35] Choudhary S P,Yu J Q,Yamaguchi-Shinozaki K,et al.Benefits of Brassinosteroid Crosstalk [J].Trends in Plant Science,2012,17(10):594-605.
[36]Clouse D, Sasse M.Brassionsteroids: Essential Regulators of Plant Growth and Development [J].Annu Rev Plant Physiol Plant Mol Biol,1998,49:427-451.
[37] 许金亮,谢鹏飞,向世鹏,等.喷施外源EBR和H2O2对低温胁迫烟苗恢复生长期生理特性的影响[J].中国烟草学报,2022,28(3):44-51.
XU Jinliang,XIE Pengfei,XIANG Shipeng,et al.Effects of exogenous EBR and H2O2 on physiological characteristics of tobacco seedlings under low temperature stress [J].Chinese Tobacco Science, 2022,28(3):44-51.
[38] Mahesh K,Balaraju P,Ramakrishna B,et al.Effect of brassinosteroids on germination and seedling growth of radish (Raphanus SativusL.) under PEG-6000 induced water stress[J].American Journal of Plant Sciences,2013,4(12): 2305-2313.
[39] 吴志勇,顾红,程大伟,等.油菜素内酯调控植物根系发育机制研究进展[J].中国农业科技导报,2022,24(2): 68-76.
WU Zhiyong,GU Hong,CHENG Dawei,et al.Advances in regulatory mechanism of brassinolide on plant root development [J].Journal of Agricultural Science and Technology,2022,24(2): 68-76.
[40] 周晔,赵璇,王璐,等.植物BZR家族基因调控非生物胁迫应答和生长发育的研究进展[J].中国油料作物学报,2020,42(4): 499-511.
ZHOU Ye,ZHAO Xuan,WANG Lu,et al.Research advances on plant BZR family genes in regulating abiotic stress response and development[J].Chinese Journal of Oil Crop Sciences,2020,42(4): 499-511.
[41] Jia D D,Chen L G,Yin G M,et al.Brassinosteroids regulate outer ovule integument growth in part via the control of inner no outer by brassinozole-resistant family transcription factors[J].Journal of Integrative Plant Biology, 2020,62(8): 1093-1111.
[42] 蘭彩耘,宋洪元.超量表达DWF4基因对芥菜生长发育的影响[J].西南大学学报(自然科学版),2021,43(12): 26-37.
LAN Caiyun,SONG Hongyuan.Effect of DWF4 Gene Overexpression on Growth and Development in Brassica juncea [J].Journal of Southwest University (Natural Science Ed.),2021,43(12): 26-37.
Effects of 2,4-epibrassinolide on growth and related genes expression of watermelon seedlings under drought Stress
ZHANG Wei1,YANG Guohui2,YU Hui1
(1. Ordos Vocational College of Eco-environment,Ordos,Inner Mongolia 017000,China; 2.College of Landscape and Horticulture,Northeast Agricultural University,Harbin 150030,China)
Abstract:【Objective】 To reveal the effects of exogenous 2,4-epibrassinolide (EBR) on the growth and related gene expression of watermelon seedlings under drought stress,so as to provide a theoretical basis for the study of watermelon drought resistance.
【Methods】 Watermelon variety Qihong 1 was taken as the experimental material by using 15% polyethylene glycol (PEG6000) to simulate drought stress,and then,three treatments were set up: CK (clear water),PEG and PEG + EBR,in which the concentration of EBR was 0.01 mg/L.Finally,the biomass,photosynthesis index,chlorophyll content,antioxidant enzyme activity and expression of related genes of watermelon seedlings after different treatments were detected.
【Results】 Spraying exogenous EBR could alleviate the inhibition and damage of drought stress on the growth and development of watermelon seedlings and also activate EBR signal transduction pathway and improve the drought tolerance of watermelon seedlings by increasing the expression of response genes.Compared with PEG stress,the plant height,root length,aboveground fresh weight and underground fresh weight of watermelon seedlings sprayed with exogenous EBR increased by 2.41%,36.76%,1.88% and 3.42% respectively.Moreover,it also enhances various indicators of photosynthesis and the activities of antioxidant enzymes (POD and SOD).The expression of key response genes BRI1,BIN2,BES1 and DWF4 of Brassinolide (BRS) signal transduction pathway also changed to varying degrees,and the expression of drought related genes CDSP32 and MYB101 also changed significantly.
【Conclusion】 Exogenous EBR can effectively alleviate the damage caused by drought stress to watermelon seedlings,thus improving the drought resistance of watermelon seedlings.
Key words:watermelon seedings; 2,4-epibrassinolide; drought stress; growth index; gene expression
Fund project:The Project of the Education Department of Inner Mongolia Autonomous Region "Preliminary Establishment of Watermelon Inbred Line Germplasm Resource Database and Character Database in Ordos Area"(NJZY19376)
Correspondence author:YU Hui (1976-),male,from Qiqihar,Heilongjiang,associate professor,master,research direction: in watermelon planting resources and breeding,(E-mail)yh1166@sina.com

