Scanning the optical characteristics of lead-free cesium titanium bromide double perovskite nanocrystals
Chenxi Yu(于晨曦), Long Gao(高龙), Wentong Li(李文彤), Qian Wang(王倩),Meng Wang(王萌), and Jiaqi Zhang(张佳旗)
Key Laboratory of Automobile Materials,Ministry of Education,College of Materials Science and Engineering,Jilin University,Changchun 130012,China
Keywords: Cs2TiBr6,nonlinear optics,ultrafast spectroscopy,absorption spectra
1. Introduction
Functional nanomaterials are of great importance for many applications.[1,2]Recently, perovskite lead halides, a new type of semiconducting nanomaterials have exhibited a very fast development in photovoltaics,[3]light-emitting diodes (LEDs),[4]photocatalysis,[5]lasers,[6]photodetectors,et al.,[7]due to their tuneable spectrum,[8]strong photoluminescence,[6]reasonable defect tolerance,[9]high diffusion coefficient,[10]high carrier mobility,[11]and long carrier lifetime.[12]However, the stability of perovskites and the toxicity of Pb currently are two major obstacles for the practical application of perovskites, which the community is trying to overcome.[13–17]Currently, two main forms are being proposed to address the toxicity of Pb, one is to use a monovalent cation and a trivalent cation to replace two divalent lead ions(B++B3+→2Pb2+)to form double perovskite formularA2B+B3+X6(e.g., Cs2AgInCl6[18–20]), the other is to use a tetravalent cation and a vacancy to replace two divalent lead ions (B4++vacancy→2Pb2+) to form vacancyordered perovskite derivative formulaA2BX6(e.g.,Cs2TiBr6).Cs2TiBrxI6-x, a perovskite derivative structure material, was firstly synthesized as powders in 2017,[21]showing benign defect properties and tuneable bandgap from 1.02 eV to 1.78 eV.Later, Cs2TiBr6was prepared as films for photovoltaic application, yet the efficiency obtained was only 3.3%.[22]So the application of the related devices is still a major challenge.Therefore, the in-depth study of relevant characteristics is of much importance for further application. Till now, most researches of Cs2TiBr6materials are focusing on the basic optical and structural properties,and mainly via Cs2TiBr6powders and polycrystalline films.[23,24]However, more profound investigation of photo-physical properties is also very important,for example,its nonlinear optical properties,carrier dynamics,and temperature-dependent energy state evolution,which will influence further application of Cs2TiBr6materials in the field of optoelectronics.
In this work, we demonstrate the photo-physical properties based on multi-spectroscopy for lead-free double perovskite Cs2TiBr6nanocrystals(NCs). The energy band structure of Cs2TiBr6NCs changes a little with temperature decreasing. Furthermore, the carrier recombination in the Cs2TiBr6·NC films, involving monomolecular and bimolecular, has been further studied by TA technique. And the nonlinear optical properties of Cs2TiBr6·NCs are investigated and the TPA section is confirmed according to the excitation intensity-dependentZ-scan technique,which excludes the interference of the excitation intensity.
2. Results and discussion
In this work, Cs2TiBr6NCs were synthesized via a hotinjection method. First, we investigated the morphology of NCs by transmission electron microscopy (TEM), exhibiting uniform, hexagonal shaped NCs with an average diameter of 13.5±1.9 nm, as shown in Figs. 1(a) and 1(b). The uniform morphology of our Cs2TiBr6NCs benefits from the precise control of the reacting temperature and modified synthesis route,compared with previous reports.[25,26]The corresponding x-ray diffraction (XRD) patterns are given in Fig. 1(c),showing (111), (220), (222), (400), (440), and (622) planes at 14.3°, 23.56°, 28.78°, 33.42°, 47.84°, and 56.84°, respectively, which is consistent with the previous report.[23]In addition, the elemental distribution was investigated by energy dispersive x-ray (EDX) analysis, presenting that the atomic ratio of Cs:Ti:Br is 20.08:9.97:69.95, as shown in supporting information (SI) Table S1, which is approximate to the ideal proportion of 2:1:6. The chemical properties were further studied by the x-ray photoelectron spectroscopy (XPS).Titanium presents as +4 valence state (Fig. 1(e)), confirming by the Ti 2p1/2and 2p3/2peaks at 459 eV and 465 eV,respectively.[27]The aforementioned results combine to reveal that the as-prepared NCs are Cs2TiBr6. The schematic crystal structure of Cs2TiBr6is shown in Fig.1(f),in which the Ti4+sites and vacancies are alternatively occupied showing ordered vacancies. And the[TiBr6]2-octahedra was isolated thus the structure can also be considered as Ti-deficient 0D perovskite.
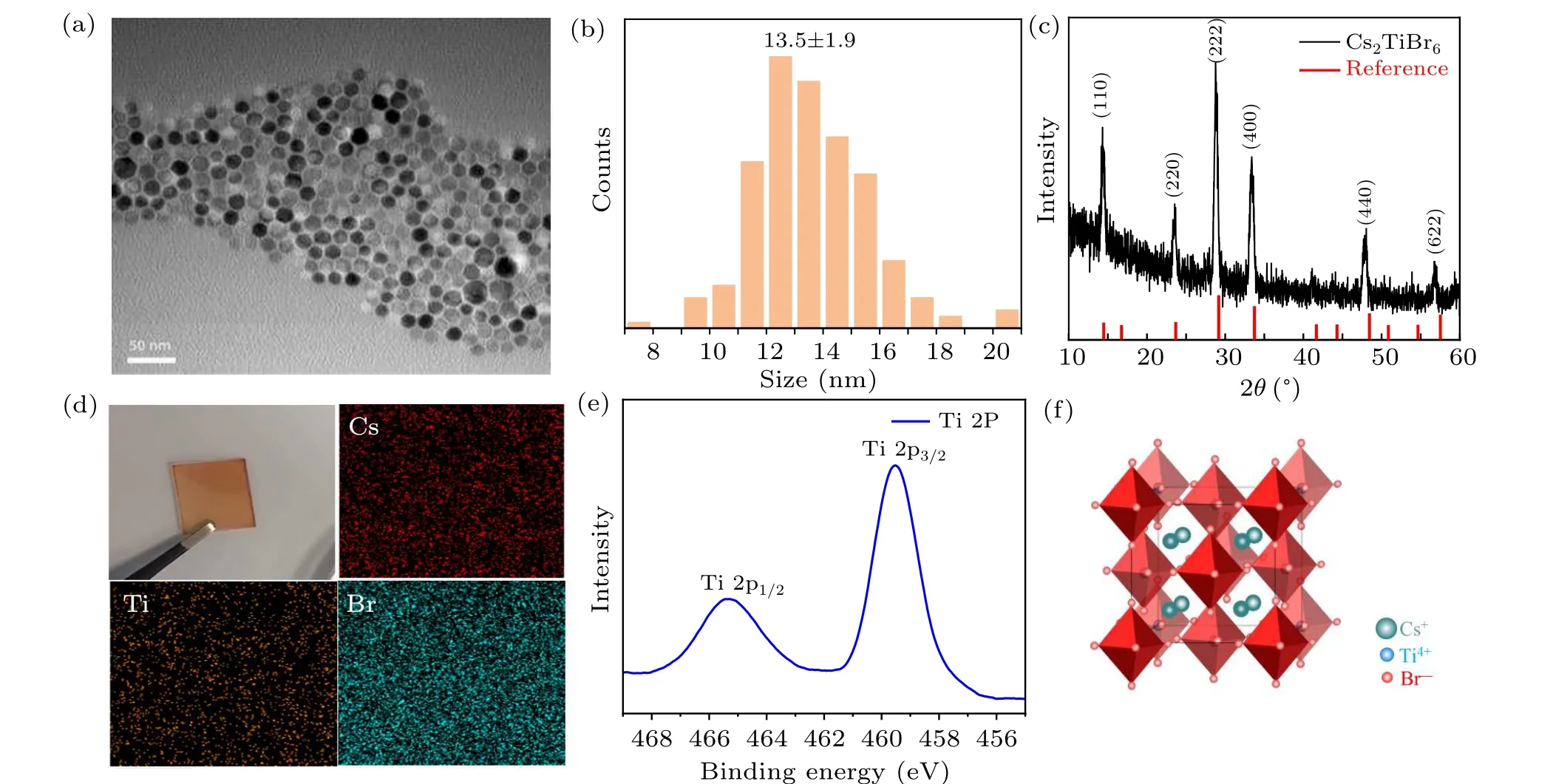
Fig. 1. (a) TEM image of Cs2TiBr6 NCs. (b) Size distribution histogram of Cs2TiBr6 NCs. (c) XRD patterns of Cs2TiBr6 NCs.[23] (d) Digital photograph of Cs2TiBr6 NCs film and EDX elemental mapping of Cs2TiBr6 NCs. (e)XPS spectrum of Ti in Cs2TiBr6 NCs. (f)Schematic crystal structure of Cs2TiBr6.

Fig. 2. (a) The temperature-dependent absorption spectra of Cs2TiBr6 NCs. (b) Tauc plot of Cs2TiBr6 NCs. (c) The changing of band edges of Cs2TiBr6 NCs with temperature increasing from 77 K to 295 K.
To study the evolution of the energy band structure of Cs2TiBr6NCs with temperature, the temperature-dependent absorption spectra of Cs2TiBr6NCs are given in Fig. 2(a).The shape of absorption spectra is almost invariable with the temperature decreasing, but the band edge shifts a little to the low-energy region simultaneously. As seen in Fig. 2(b),the bandgap of Cs2TiBr6NCs can be estimated based on Tauc plots with indirect bandgap type. TheEgvalue is about 1.85 eV at 77 K and about 1.82 eV at 295 K. Compared to the previous reports about Cs2TiBr6bulk powers and polycrystalline films, our NCs have similar bandgap at room temperature.[21,28]Thus our Cs2TiBr6NCs with a size of 13 nm are considered to have a negligible quantum effect.The band edges of Cs2TiBr6NCs as a function of temperature are summarized in Fig. 2(c), showing that all of them almost linearly shift to the low energy region as the temperature lowers from 295 K to 77 K.As the temperature was varied about 220 K,the bandgap changes about 30 meV,implying that the energy band structure is almost invariable.
Actually, the intrinsic absorption properties of Cs2TiBr6NCs can also be investigated by femtosecond transient absorption(fs-TA)spectroscopy techniques.[29,30]Herein,figure 3(a)shows the time-dependent TA spectrum of Cs2TiBr6NCs at room temperature, in which a strong ground state bleaching(GSB) signal is located at~481 nm, meanwhile two photoinduced absorption (PIA) bands are located at 575 nm and 448 nm, respectively. The PIA signal at 575 nm narrows noticeably with time prolonging in the low energy region,compared to the GSB signal, where the change is less obvious.Figure 3(b)shows the TA spectrum of Cs2TiBr6NCs at 77 K,which is also composed of a small PIA at 440 nm, a GSB at 493 nm and a PIA at 560 nm. Apparently, the shift of the signal is assigned to the variance of the energy structure.
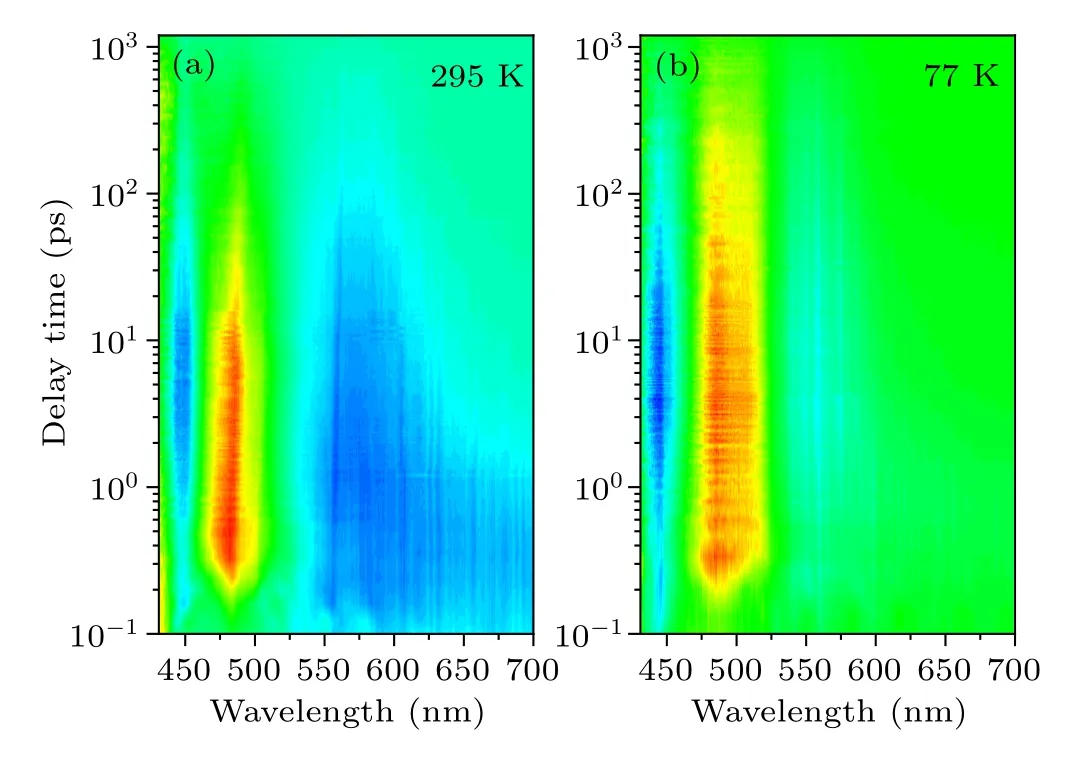
Fig. 3. The time-dependent TA spectra of Cs2TiBr6 NCs (a) at room temperature and(b)at 77 K.
Considering that Cs2TiBr6NCs have a huge potential in the optoelectronic field,[31]the understanding of carrier recombination dynamics is of much importance.[32]The TA curves of Cs2TiBr6NCs as a function of excitation intensity were probed at room temperature and 77 K (as seen in Figs. 4(a) and 4(b)). Herein, the Cs2TiBr6NC films in our experiment were all prepared by drop-casting on a glass substrate and subsequently permitting the solvent evaporation at room temperature under inert gas environment.[33]The pump flux-dependent bleaching signal of Cs2TiBr6NCs at room temperature and 77 K is given in Figs. 4(c) and 4(d).Based on Poisson statistics,[34]the absorption cross sections of these Cs2TiBr6NCs at 400 nm can be extracted,which are 3.74×10-13cm2at room temperature and 5.60×10-13cm2at 77 K.Apparently, the absorption coefficient was enhanced with the temperature decreasing. Compared with lead halide perovskite(MAPbBr3),the absorption cross section is one order of magnitude higher.[35]Simultaneously, Cs2TiBr6NCs process a higher absorption coefficient than double perovskite Cs2AgInxBi1-xCl6(x= 0,0.75) and Cs2AgSb0.25Bi0.75Br6NCs are much better than CsPbBr3perovskites.[36–41]Note that the GSB curve only exhibits a simple exponential relaxation behavior at low excitation intensity when the temperature is 295 K(room temperature). After the pump fluence exceeds 4.08×1013photon·cm-2, an excitation intensity-dependent rapid relaxation component gradually appears in the GSB curves,causing the GSB curves exhibiting an apparent multiexponential relaxation behavior. Moreover, its weight seems to enhance with the excitation intensity.[42–46]The similar phenomenon was observed at 77 K.Based on the analysis of TA data, it is found that the recombination rate constants can be quantitatively ascertained by employing the polynomial rate equation[32]

wherendenotes the initial carrier density;k1andk2are the monomolecular and bimolecular recombination rate constants,respectively. In other words, the Auger recombination process did not appear in the GSB curves,even though the intensity has increased to 13.41×1013photon·cm-2. This suggests that the carrier diffusion velocity in Cs2TiBr6NCs film is really rapid, which can restrict the happening of Auger recombination. After fitting, these recombination rate constants of Cs2TiBr6NCs can be extracted from the excitation intensitydependent TA curves and summarized in Figs. 4(c) and 4(d).As the temperature decreases to 77 K, the monomolecular recombination constantk1apparently decreases from 9.5×109s-1to 6.2×109s-1, meanwhile the bimolecular recombination constantk2increases from 0.9×10-9cm3·s-1to 3.1×10-9cm3·s-1. Generally speaking,k2of Cs2TiBr6NCs film is smaller in comparison with other conventional perovskite materials,[19,45,47,48]suggesting that the loss originated from bimolecular recombination in devices based on Cs2TiBr6NCs should be less than that based on other perovskite materials. According to the previous report,[49]the thermally activated charge trapping model can be responsible for this phenomenon. As the temperature decreases,the activity of carriers gradually weakens,compelling the carriers to spend much time overcoming the energy barrier and recombining with the shallow defects. In this situation, the monomolecular recombination constant has to decrease with temperature dropping.Meanwhile, the carriers left in the intra-band apparently increase, which enhances the bimolecular recombination constant. Moreover, the decreasing temperature can lead to the shrinkage of the lattice and up shift the distribution of defects,which restricts the capture of carriers originated from the defects.This can also restrict the monomolecular recombination.On the other hand,the decreasing temperature restricts the vibration of the lattice and weakens the carrier–phonon scattering,which facilitates the migration of carriers and accelerates the monomolecular recombination simultaneously.

Fig.4. The Cs2TiBr6 NCs TA(dynamic)curves with excitation at 480 nm at room temperature(a)and 77 K(b). The pump flux-dependent bleaching peak of Cs2TiBr6 NCs at room temperature(c)and 77 K(d). The monomolecular and bimolecular recombination rate constants(e)k1 and(f)k2.
Besides the temperature-dependent energy band and carrier properties, the traditional perovskite NCs often exhibit excellent two-photon absorption (TPA) properties and have a huge potential in the field of conventional electronic switches.[50]Herein, the TPA properties of Cs2TiBr6NCs were measured by excitation intensity-dependent open-holeZ-scan technique,[51,52]as shown in Fig. 5, showing that the valley of Cs2TiBr6NCs is estimated to be 1.34×10-3M,according to the linear absorption coefficient. The scattered data and systematic asymmetry in baselines are usually caused by laser instability and surface imperfection and can be corrected by the low intensity background responses. Moreover,the contribution from the solvent has been excluded in our experiment. The obtained data clearly indicates a reverse saturable absorption type of behavior and the depth of valley is apparently enhanced with the excitation intensity. By fitting theZ-scan curves,the two-photon absorption coefficients(β)of Cs2TiBr6NCs were measured and shown as the inset of Fig.5,which is almost independent of the excitation intensity.Finally,the two-photon absorption(TPA)cross section(σTAP)of Cs2TiBr6NCs is estimated to be 1070 GM, which is apparently larger than that of other typical perovskite bromide materials.[53]Apparently, Cs2TiBr6NCs can provide broad bandwidth unattainable by conventional electronic switches owing to their nonlinear optical properties.
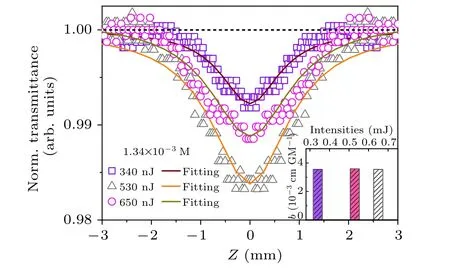
Fig.5. Excitation intensity dependent Z-scan curves of Cs2TiBr6 NCs. Inset: β at different excitation intensity.
3. Conclusion and perspectives
In summary,we report on the synthesis of lead-free double perovskite Cs2TiBr6NCs and confirm its photo-physical properties based on multi-spectroscopy.It is interesting to find that the energy band structure of Cs2TiBr6NCs changes a little with temperature decreasing. In addition,the carrier recombination, involving monomolecular and bimolecular, occurring in the Cs2TiBr6·NC films has been further investigated by TA technique, indicating that the monomolecular recombination decreases with the temperature lowing,yet the bimolecular recombination shows opposite tendency. Moreover,the nonlinear optical properties of Cs2TiBr6·NC are studied and its TPA section is estimated to be 1070 GM based on the excitation intensity-dependentZ-scan technique,which excludes the interference of excitation intensity. This provides a comprehensive insight into the photo-physical properties of double perovskite NCs, suggesting that this type of perovskite nanomaterials have great potential in the fields of conventional electronic switches.
Acknowledgements
Project supported by the National Natural Science Foundation of China (Grant No. 61804063) and the Natural Science Foundation of Jilin Province, China (Grant No.20190201208JC).
- Chinese Physics B的其它文章
- A nonlocal Boussinesq equation: Multiple-soliton solutions and symmetry analysis
- Correlation and trust mechanism-based rumor propagation model in complex social networks
- Gauss quadrature based finite temperature Lanczos method
- Experimental realization of quantum controlled teleportation of arbitrary two-qubit state via a five-qubit entangled state
- Self-error-rejecting multipartite entanglement purification for electron systems assisted by quantum-dot spins in optical microcavities
- Pseudospin symmetric solutions of the Dirac equation with the modified Rosen–Morse potential using Nikiforov–Uvarov method and supersymmetric quantum mechanics approach

