Computational design of ratiometric two-photon fluorescent Zn2+probes based on quinoline and di-2-picolylamine moieties
Zhe Shao(邵哲), Wen-Ying Zhang(张纹莹), and Ke Zhao(赵珂)
School of Physics and Electronics,Shandong Normal University,Jinan 250358,China
Keywords: two-photon absorption,fluorescent probe,zinc ion,intra-molecular charge transfer
1. Int roduction
Two-photon absorption (TPA) active molecules can be used as fluorophores to form fluorescent probes which have important applications in two-photon microscopy(TPM).[1]In comparison with one-photon microscopy,TPM has several advantageous features including higher spatial resolution,deeper penetration depth, lower tissue autofluorescence and selfabsorption,less photobleaching and photodamage,and longer observation time.[2—4]Nowadays, great efforts have been exerted for the design and synthesis of two-photon (TP) fluorescent probes with desirable sensing properties for diverse analytes[5—12]based on various recognition mechanisms,such as photon-induced electron transfer (PET), intra-molecular charge transfer (ICT), fluorescent resonance energy transfer,aggregation-induced emission,and so on.[13—16]
Zinc ion is an essential component in enzymes and proteins and involves in many biological processes.[17,18]As such,it is highly required to develop effective fluorescent probes for detecting zinc ions in living system. In recent years, a lot of turn-on or ratiometric TP fluorescent probes for zinc ions have been synthesized based on PET or ICT mechanisms.[5,6]Superior to turn-on probes,ratiometric probes can provide quantitative detection of the analyte by measuring the ratio of emissions at two different wavelengths.[6]Therefore, there is a strong need for the development of ratiometric TP zinc ion probes.
Theoretical study could reveal the structure-property relationships and provide guidelines for the design of functional materials with improving properties.[19—26]At the same computational level,the optical properties of different compounds can be compared accurately. However, contrast to great experimental works,the theoretical study of TP zinc ion probes is still very limited.[27—33]Ren and co-workers[27]designed a series of Salen-based TP zinc ion probes based on ICT mechanism. The one-photon absorption (OPA), TPA and fluorescence emission properties were studied by the quantum chemical calculations. They also performed a theoretical investigation on bipyridine-centered ratiomatric zinc ion probes.[28]Zhanget al.[29]elucidated the response mechanisms for a series of 2-(2’-hydroxyphenyl)benzoxazole-containing zinc ion probes by means of response theory. Employing the same computational methods,they also studied a series of TP fluorescent probes for mercury ions.[30]Their work indicated that response functions and density functional theory (DFT) can successfully be used to analyze metal ion probes. In our previous work,[31]response theory was used to investigate OPA,TPA as well as emission properties of a series of zinc ion probes. Special interests focused on the coordination mode effects.[32,33]
Recently,Liet al.synthesized a new ICT-based TP probe for ratiometric imaging and biosensing of Zn2+in living cells.[34]The developed probe has good water solubility and excellent sensitivity and selectivity for ratiometric determining of Zn2+. However, the maximum TPA cross section values were a little lower than the previously reported probes.[34]To improve TPA properties,in this work,we designed a series of ratiometric TP Zn2+probes based on the newly synthesized probe according to ICT mechanism. In the first design,several acceptors or donors were employed to increase the electron accepting or donating ability,accompanying by the change of conjugated length and the dimensionality of charge transfer channel. The OPA and TPA properties of the experimental and designed probes before and after combination with Zn2+were computed at DFT level. The structure-property relationships were analyzed. Then,we performed a second design by picking out the most efficient acceptor and donor from the first design. The TPA property of the second designed probe was calculated and compared with experimental system. At last,the fluorescence properties of the designed probes were computed and evaluated for ratiometric detection. The aim of the design is to achieve the TP probes having not only the large TPA cross section but also strong fluorescence intensity with the significant shift in emission spectrum upon Zn2+coordination.Our research will provide useful guidelines for the design and synthesis of ratiometric TP probes for metal ions.
2. Computational method
In this study, the formulas for calculation of OPA and TPA properties were same as our previous works.[31,32]The 6-31G(d)basis set and the B3LYP hybrid functional were used to optimize the geometries of ground states. On the optimized structures, the OPA properties were computed by the timedependent DFT(TD-DFT)approach using the same functional and basis set. The geometry optimization of the first excited state and fluorescent emission properties were also calculated using TD-DFT methods at the same level. All the calculations mentioned above were carried out in the Gaussian 16 program.[35]The TPA cross sections were computed by response theory using the B3LYP functional with 6-31G(d)basis set in the Dalton 2013 package.[36]In addition, the effect of methanol solvent is considered by means of the polarizable continuum model in both Gaussian and Dalton calculations.Our previous work has shown that the B3LYP functional calculations can give reasonable TPA properties which are consistent with the trend of experimental observations.[24—26,31,32]
3. Results and discussion
3.1. First design and geometry optimization
The chemical structure of the model probe 1 is depicted in Fig.1. Probe 1 uses the 1-(pyridin-2-yl)-N-(pyridin-2-ylmethyl)-N-(quinolin-2-ylmethyl)methanamine moiety as Zn2+chelator. The quinoline unit can act both as an electron acceptor of the fluorophore and as a recognition site for Zn2+. The four nitrogen atoms from the quinoline and di-2-picolylamine (DPA) could coordinate with Zn2+by donating lone electron pairs into empty metal orbitals. Because the Zn2+, which has electron withdrawing ability, can connect with the acceptor quinoline group,the ICT from electron donor to acceptor will enhance upon binding with Zn2+,thus improving the TPA intensity as the experiment observed.
To enhance the TPA intensity, one needs to consider the existing structure and TPA property relations. It has been revealed that some structural factors, such as the electron richness and planarity of theπ-conjugation center, the molecular symmetry, the strength of donor and acceptor, as well as the conjugated length and dimensionality, have important effects on TPA property.[19—21]On the basis of the model probe, at first,we designed three new structures 2,3,and 4 by increasing the strength of acceptor, as shown in Fig.1. It is well known that triazine or diazine group has strong electron-accepting ability and has been often used as the center of multibranched TPA active chromophores.[37]Hence, the quinoline unit is replaced by quinazoline in probe 2 to increase the electronaccepting ability. In probe 3,a cyano group,which is a common acceptor, is added to the quinoline group to increase the acceptor strength. The following acceptor which comes into our mind is the acyl moiety. It is a very flexible acceptor and can also get involved in the Zn2+coordination together with DPA.[38]In our previous work,[31]we have found that the receptor containing acyl and DPA moieties can give rise to higher TPA cross section than the one consisting of pyridine and DPA.As such,an acyl unit is inserted between quinoline and DPA in probe 4. The introduction of acyl moiety can not only strengthen the acceptor, but also extend the conjugated length, which would like to improve TPA response. Then,based on probe 1, we designed another two probes 5 and 6 by increasing the electron-donating ability. The chemical formulas are also presented in Fig.1. In probe 5,two methyl terminals are substituted by two amino groups,while,in probe 6,the methoxy terminal is replaced by a dimethylamino group because the dimethylamino has stronger ability to donate and transport electrons. Although only one or two functional groups are changed or added in these designed structures to enhance the acceptor or donor strength,the charge transfer dimension, the conjugated length, and the type of coordination bond could also be changed at the same time. Therefore, it would be very interesting to compare the structures and TPA properties of these probes and find which design way is the most effective one to enhance TPA.
The optimized geometries of these six probes and their zinc complexes are given in Fig. 2. The frequency calculations for all the geometries do not produce any imaginary frequencies. Two Cl-ions are included explicitly to consider the counter ion effects.[31,39]It is noticed that all the backbones of fluorophore have good planarity. Four coordination bonds can be formed in all the zinc complexes. The Zn2+can chelate both the DPA receptor and the acyl moiety in 4Zn complex.The coordination bond length has the typical value of 2.1 °A—2.3 °A,which is similar to the x-ray crystal data.[38]The structures of 3 and 3Zn are bended a lot due to the steric effect of cyano group. While in 4 and 4Zn, the added acyl moiety leads to a more linear backbone. It should be mentioned that the introduction of the cyano group could affect the binding sites of Zn2+ions for 3Zn. The Zn2+ions could coordinate with the nitrogen atom of the cyano group. We also optimized 3Zn using different initial conformations, but the convergent geometry in which the zinc ion coordinates with both the DPA receptor and the cyano group was not obtained.
The calculated permanent dipole moments and dihedral angles for these structures are presented in Table 1. It shows that the permanent dipole moments of zinc complexes are much larger than those of free ligands. The values of 4 and 4Zn are the largest ones among both free ligands and zinc complexes. The planarity of the backbone can be described by the dihedral angle of two phenyl ringsφ. It is noticed that most of the structures have nearly planar backbones and theφchanges from about 165°to 180°. Theφof 6 and 6Zn are equal to 166.13°and 178.47°, respectively. The difference is a little larger than other probes upon binding with zinc ion.

Table 1. Values of permanent dipole moment μ and dihedral angle φ.
3.2. One-photon absorption
The experimental UV-vis absorption maximum of probe 1 appeared at 360 nm with a broad band. With addition of Zn2+,the absorption increased at 430 nm.[34]Our calculated OPA wavelengthλopand oscillator strengthfopof all the studied molecules are listed in Table 2. One can see thatλopof probe 1 is located at the first excited state S1with 373 nm, which is close to the value of experiment. The third excited state S3also has considerablefop. This means there is another absorption peak at 333 nm with lower intensity. When the molecule binds to Zn2+, both the first and second excited states have strong absorption. The two absorption bands are red-shifted in comparison with the free ligand. This is consistent with the experimental observations. With respect to probe 1,theλopof the S1for all the designed probes is red-shifted significantly because of the stronger acceptor or donor. The values of 3,4,and 6 come to above 400 nm,which indicates the fluorophores in these molecules are more beneficial to charge transfer. It should be mentioned that the TP probe that can be excited at longer wavelength can increase penetration depth and lower tissue autofluorescence and photobleaching.[3]From Table 2,one can notice that upon Zn2+binding,λopof the S1has a large red-shift for all the designed probes. The red-shift of 4Zn is the largest one and comes to 40 nm. All the structures have more than one absorption bands with different intensities except 4 and its zinc complex 4Zn. In 4 and 4Zn,only the S1state has a very strongfop. This is because 4 and 4Zn have good linear skeletons.
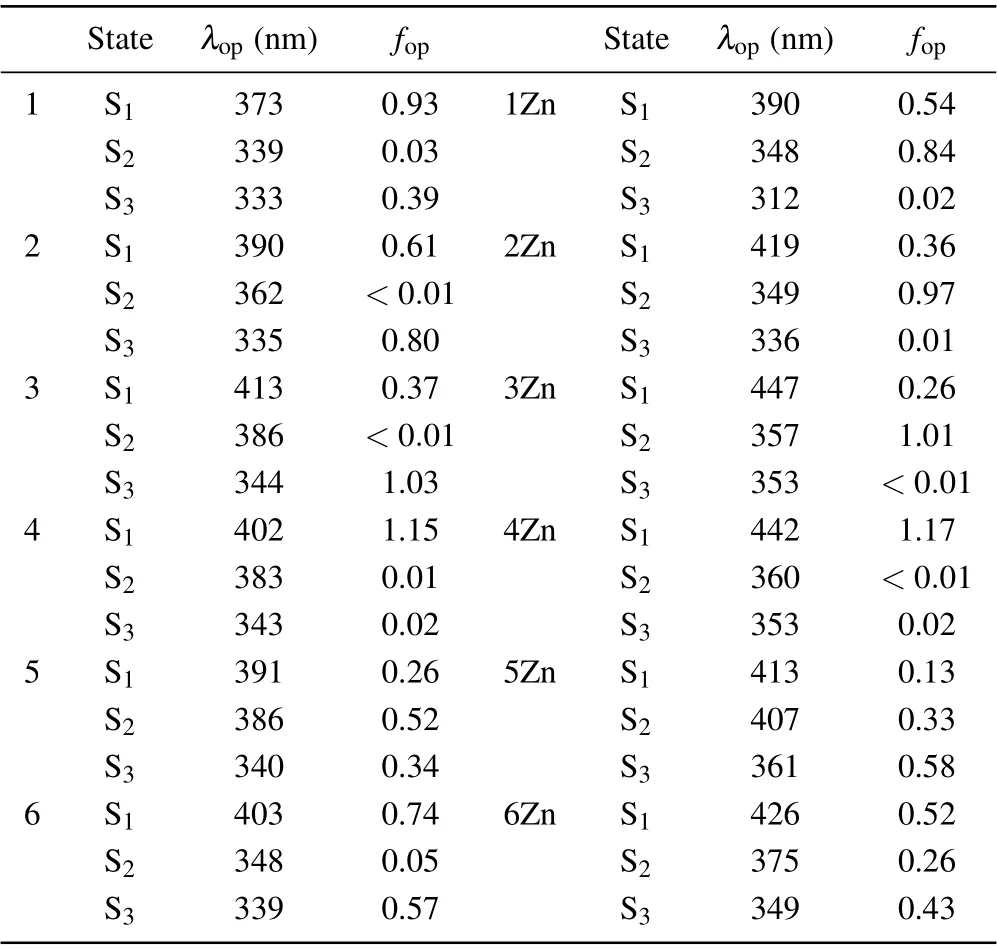
Table 2. One-photon absorption properties of the molecules in methanol solvent.
The energy gaps between the highest occupied molecular orbital(HOMO)and the lowest unoccupied molecular orbital(LUMO)of these molecules are calculated and the results are illustrated in Fig. 3. One can see that the energy gaps for all the designed probes are lower than the probe 1. Moreover,the values decrease as the zinc complexes are formed. For example,when the probe 4 combines with Zn2+,the energy gap decreases significantly,from 3.40 eV to 3.10 eV.The relatively smaller energy gaps of the zinc complexes compared with corresponding free ligands lead to the red shift of the OPA. 4Zn has the greatest decrease in energy gap, and thereby its red shift is the largest one among zinc complexes. These results indicate that the ICT increases in the designed probes and the OPA process takes place more easily upon binding with Zn2+.
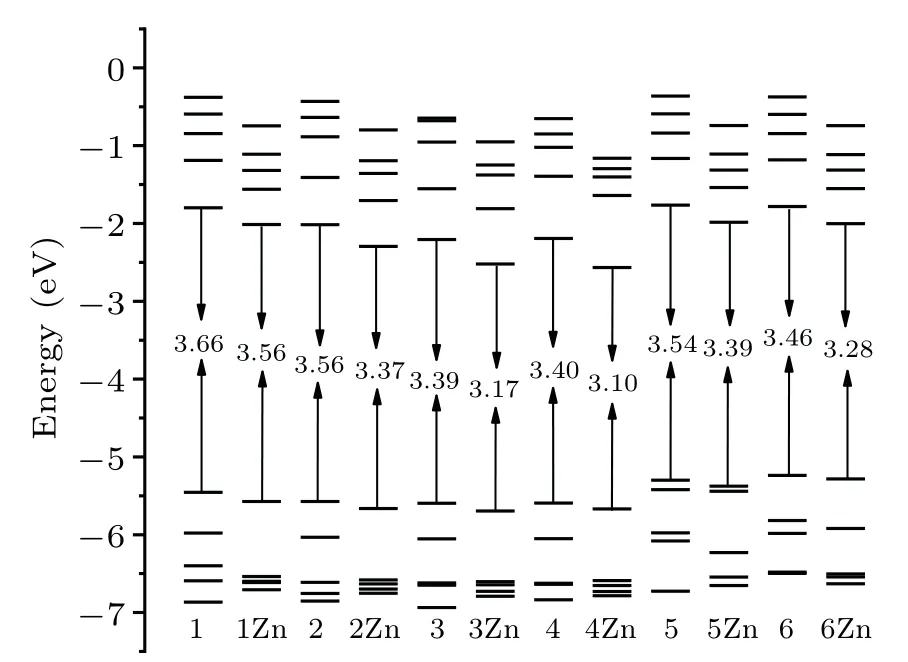
Fig.3. Frontier orbital energy levels.
3.3. Two-photon absorption
The TPA wavelengthλtpand cross sectionσfor all the studied molecules in methanol solvent have been calculated by the response theory in Dalton program. The TPA properties of the three lowest excited states are presented in Table 3.For probe 1,the first excited state S1has the largestσ,which is equal to 46.0 GM (1 GM=10-50cm4·s/photo) at 738 nm.Besides the S1,the third excited state S3also has comparable cross section. When the probe coordinates with Zn2+,theλtpof S1shifts to 778 nm and the correspondingσincreases up to 106.0 GM. Theσof S2is much smaller than that of S1.In experiment, the peak of TPA spectrum is red-shifted from about 720 nm to 750 nm and the cross section is increased from 115 GM to 188 GM after the probe was chelated with Zn2+.[34]Therefore,our TPA calculations agree well with the trend of experiment.

Table 3. Two-photon absorption wavelengths λtp and cross sections σ of the three lowest excited states of the molecules in methanol solvent.
For the designed probes 2 and 3, both S1and S2have comparable TPA intensities. The largestσof 2 and 3 are 92.9 GM at 770 nm and 99.6 GM at 819 nm,respectively. Theλtpis red-shifted greatly and theσhas about two-fold increase with respect to probe 1. These demonstrate that the substitution of quinazoline and the addition of cyano can increase the ICT and result in TPA enhancement as our expectation. Although theλtpof probe 3 is much longer than that of probe 2,the largestσis only slightly higher. This is probably because the probe 3 has more bended backbone from the structure point of view. As for linear probe 4, the TPA mainly focuses on the S1and theσrises to 360.8 GM,much higher than that of probe 1. This implies that the addition of acyl unit is a very efficient way to improve TPA intensity. It not only makes the acceptor strength stronger,but also brings about longer conjugated length and more linear ICT channel. Therefore,one can realize that various structural factors need to be considered simultaneously for designing TP probes. When the two methyl terminals are substituted by two amino groups, probe 5 has a largestσof 137.5 GM at the S2, 768 nm. It is known that the selection rules of one-photon and two-photon transitions are different. The transition between two states with opposite parities are allowed in one-photon process,while same parities are necessary for TPA.The state parity is directly related to the structural symmetry. For a typical symmetric charge-transfer molecule, the second excited state S2usually is the most active TPA state.[24]In probe 5, because the two amino groups increase the symmetry of the charge transfer channel, the S2produces the largest cross section.In comparison with probe 5,probe 6 has a strongerσof 252.7 GM at 790 nm. This indicates that enhancing the electron-donating ability along the line of backbone can improve TPA intensity more effectively.In a word, all the designed probes give rise to stronger TPA responses at longer wavelengths in comparison with probe 1,which confirms our design strategies.
Upon binding with Zn2+, the largestσfor all the zinc complexes increases significantly and the correspondingλtphas a great red-shift. The largestσof 2Zn rises to 148.0 GM at 838 nm,which has a red-shift of 68 nm with respect to free ligand 2. As for 3Zn,the shift ofλtpcomes to 80 nm and theσof the S1becomes to 185.1 GM, much larger than that of probe 3. 4Zn has the largest shift ofλtplike the case of OPA.The red-shift reaches up to 88 nm. The much lower excitation energy makes theσof 4Zn enhanced greatly. Theσgrows to 844.6 GM, more than two times of probe 4. It is also about eight times of zinc complex 1Zn. This demonstrates again that the introduction of an acyl unit can improve TPA intensity very effectively. The receptor consisting of acyl and DPA moieties is beneficial for improving TPA upon coordination with Zn2+.In the case of 5Zn,two strong absorption states appear at about 824 nm and 730 nm with the cross sections of 184.7 GM and 105.3 GM, respectively. The S2gives the largest cross section, same as the case of probe 5. While in 6Zn, theσat 847 nm is 331.8 GM,much larger than that of 5Zn. Therefore,the dimethylamino terminal is better for enhancing TPA intensity. It is noticed that the S3of 5Zn and the S2of 6Zn also have considerable TPA intensities,which means the change of donor results in more than one absorption bands in the zinc complexes.
3.4. Second design
According to the results of the first design,we have found that the addition of the acyl unit is the most efficient way to increase electron accepting ability and the replacement by one dimethylamino terminal is better than the change of two amino groups for improving TPA intensity. Therefore, in the second design, we construct a new structure using both the acyl unit and the dimethylamino terminal to increase acceptor and donor strengths at the same time. The chemical structures and the optimized geometries for the second designed probe 7 and the corresponding zinc complex 7Zn are shown in Fig.4.
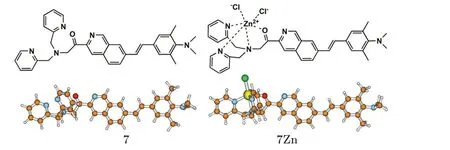
Fig. 4. Chemical structures and optimized geometries of probe 7 and the corresponding zinc complex 7Zn.
As it is expected, the calculated largestσof 7 and 7Zn rise to 727.8 GM at 874 nm and 1334.2 GM at 997 nm, respectively. The TPA cross section of 7Zn increases one-order of magnitude with respect to 1Zn and the wavelength comes to above 900 nm. As we know, there is a strong need to develop the TP probe that can be excited at longer wavelength(>900 nm). For a good comparison,the simulated TPA spectra for the probe 1,4,7,and their zinc complexes are plotted in Fig.5. It shows that the spectra of all the structures have one strong TPA peak at different positions. Compared with the experimental probe 1,the absorption intensities of probes 4 and 7 enhance greatly. Upon zinc ion coordination,the absorption wavelength of 4Zn and 7Zn are red-shifted a lot and the absorption intensities are raised about two times with respect to their free ligands.The peak of 7Zn appears at around 1000 nm and the absorption intensity reaches up to 1334.2 GM.Using an acyl unit and a dimethylamino terminal can give rise to a very large increase of TPA cross section due to the enhanced both acceptor and donor strengths.
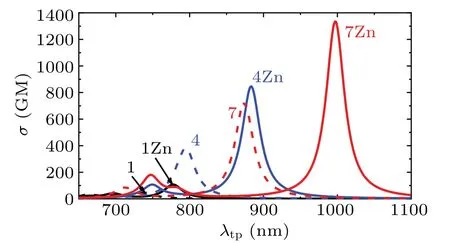
Fig.5. TPA spectra of probes 1,4,7 and the corresponding zinc complexes.
The molecular orbitals that are involved in the strong TPA excitations are also analyzed. The prominent TPA excitation of probes 1 and 7 is the result of the HOMO→LUMO transition. The related orbitals are plotted in Fig.6. In probe 7,the charge is transferred obviously from the donor dimethylamino terminal to the acceptor acyl moiety. While in probe 1, the charge is transferred very slightly. This indicates that the ICT of the designed probe 7 is stronger than that of experimental probe 1.Therefore,the designed probe 7 has much higher TPA intensity.[40—42]
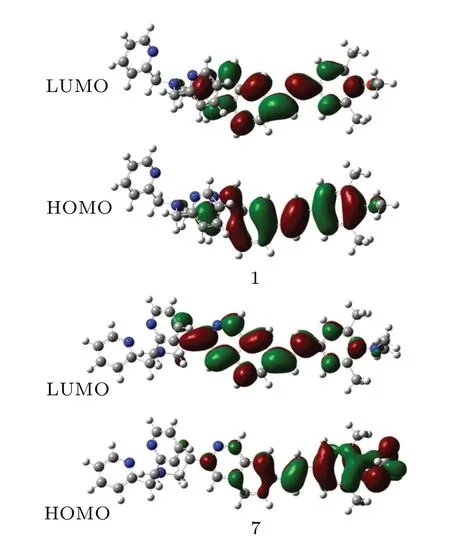
Fig.6. HOMO and LUMO orbitals of probes 1 and 7.
3.5. Emission property
For ratiometric detection, large difference of emission wavelength between free ligand and zinc complex is necessary.Hence, the emission properties of all the studied molecules have been calculated. The geometry optimization for the first excited state were carried out by the TD-DFT method at B3LYP level with 6-31G(d) basis set in methanol solvent.The obtained fluorescence emission wavelengthλemand corresponding oscillator strengthfemare given in Table 4.
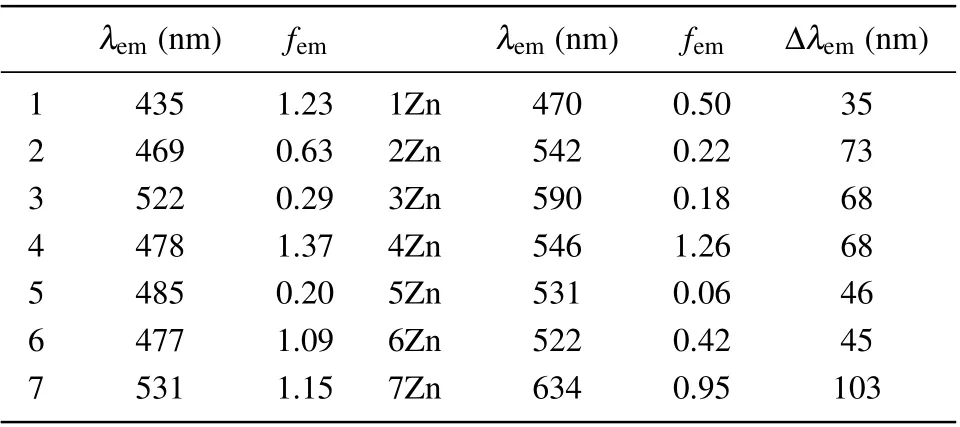
Table 4. Values of emission wavelengths λem,oscillator strengths fem,and the difference of emission wavelengths Δλem between the free ligand and the corresponding zinc complex in methanol solvent.
Theλemof the probe 1 is 435 nm, which tends to the value of experiment, 455 nm. From free ligand to zinc complex,λemof 1Zn is red-shifted to 470 nm. The difference of emission wavelength Δλemis 35 nm. This is consistent with the trend of the experimental data. The oscillator strengthfemdecreases as the probe 1 coordinates with Zn2+, which indicates that the fluorescent intensity is reduced. It exhibits that Δλemof all the designed probes is larger than that of probe 1.For probes 4 and 7, Δλemextends to 68 nm and 103 nm, respectively. Moreover, their oscillator strengths are quite high before and after coordination with Zn2+.
An effective TP probe should have significant TPA action cross section,which is the product of the TPA cross sectionσand the fluorescence quantum yieldφF. It is essential to optimize both values to develop TP fluorophores for imaging applications. It has been known that the quantum yield has relations to some molecular descriptors. Yamaguchiet al.[43]investigated the photophysical properties of conjugated and fused aromatic hydrocarbons and examined correlation between theφFand magnitude of theπconjugation length in the first excited state.[43]Recently, Humbert-Drozet al.[44]performed a high-level calculations for substitutedπconjugated terpyridine derivatives and confirmed that theφFshowed a global S-shape dependence on the magnitude of the charge separation, which can be quantified by the change in dipole moments between the ground and excited states. Their work provides a remarkable tool to predictφFforπconjugated molecules with strong charge transfer character of the first excited state.
Because the compounds studied by Humbert-Drozet al.[44]are also linear donor—acceptor-type probes and have similar characters including the molecular elements and sizes to our designed fluorophores, at first, we choose the compounds in their work,[44]together with the model probes 1 and 1Zn, to construct the relation between the measured fluorescence quantum yield and the computed difference of dipole moment. The experimental fluorescence quantum yields of the probe 1 and its zinc complex 1Zn are 0.16 and 0.22,respectively.[34]We calculated their dipole moments of the ground state and the first excited state by TD-DFT method using CAM-B3LYP functional.[44]The lengths of the vector difference of dipole moment Δμare obtained and the values are 0.7343 e·°A and 1.3176 e·°A.The data of the experimentalφFand the calculated Δμfor probes 1 and 1Zn,and the compounds 1a—1e and 1g,which are taken from Ref.[44],are presented in Fig.7. Their relation can be fitted by the exponential function as[43,44]

whereAandBare the fitting parameters. To our pleasant surprise,the points of 1 and 1Zn are nearly located on the fitting line, which is almost same as the one obtained by Humbert-Drozet al.[44]This demonstrates that the relation is very suitable and reliable for our studied systems. The relation shows theφFhas a nearly linear dependence on Δμin a certain range.
Then, according to the obtained relation, the Δμand theφFare calculated for the designed probes 4 and 7, and their zinc complexes. The results are given in Table 5. TheφFof 1 and 1Zn are also calculated for comparison.It is found that theφFof 1 and 1Zn are in a very good agreement with experimental data. TheφFof the probes 4 and 7 increase to a great extent with respect to probe 1. TheφFof probe 7 is about twice as high as the probe 1. TheφFof the zinc complexes 4Zn and 7Zn are both a little larger than those of their free ligands 4 and 7, which is consistent with experimental observations on probe 1.Finally, TPA and fluorescence properties are considered together and the TPA action cross sectionφFσare computed(see Table 5). TheφFσof the designed probes 4 and 7 are enhanced dramatically, which demonstrates that they have very strong TP excited fluorescent intensity and are expected to have superior performance for imaging applications. Our design not only improves the TPA cross section, but also enhances the fluorescence quantum yield.
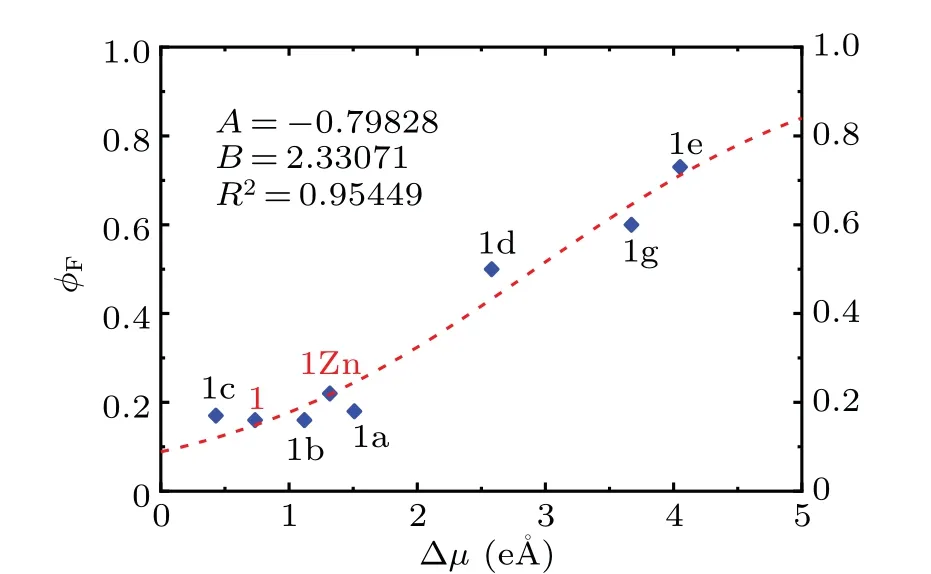
Fig. 7. The experimental fluorescence quantum yield φF as a function of calculated difference of dipole moment Δμ for probe 1 and its zinc complex 1Zn,and the compounds studied in Ref.[44].The dotted line corresponds to the fit obtained with Eq.(1).The fitting parameters A and B,and the squared correlation coefficient R2 are provided.

Table 5. The lengths of the difference of the dipole moments between the ground state and the first excited states Δμ,the fluorescence quantum yields φF,the TPA cross sections σ,and the TPA action cross sections φFσ.
It should be noticed that all the probes could have various isomers which might produce different fluorescent properties. The effect of isomerism needs to be considered. Starting from different initial configuration,the geometry optimization of probe 1 gives other four equilibrium conformations 1ISa-1ISd, as shown in Fig. 8. In much the same way, four isomers 7ISa—7ISd are also obtained for probe 7(Fig.8)It can be seen that these isomers differ by the orientations of pyridines in DPA groups.
The Δμand theφFare calculated for the various isomers and the results are listed in Table 6.The various conformations of probe 1 give rise to nearly sameφF, which is very close to the experimental value 0.16. In the case of the designed probe 7,the difference ofφFis also very small(<0.03). Furthermore, all theφFare above 0.3, much higher than that of probe 1. This indicates that the isomerism has a trivial effect on the fluorescence quantum yield,and the designed probe is expected to have stronger fluorescent intensity.
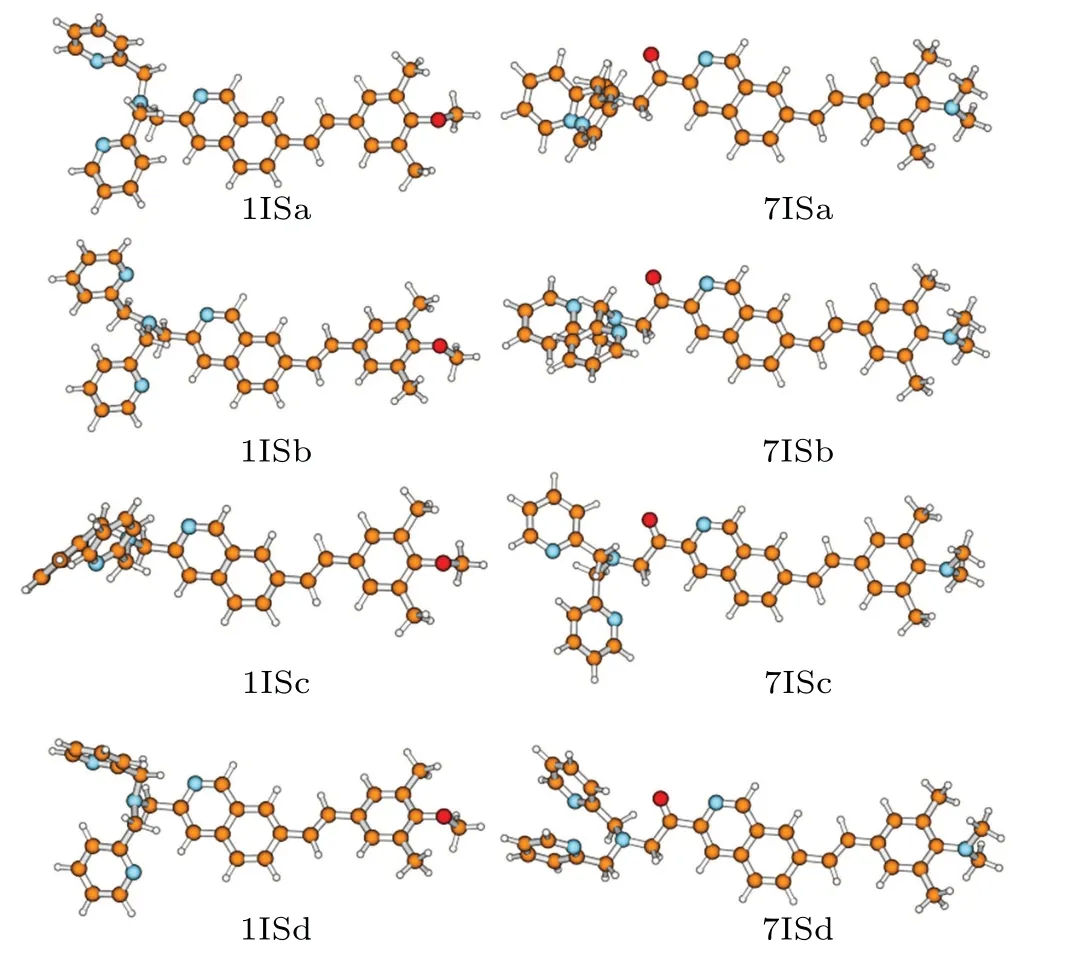
Fig.8. Optimized geometries of the various isomers for probes 1 and 7.
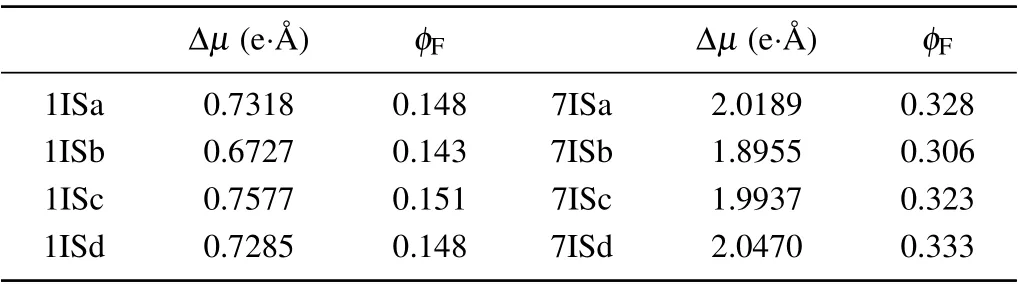
Table 6. The lengths of the difference of the dipole moments between the ground state and the first excited states Δμ and the fluorescence quantum yields φF of the various isomers for probes 1 and 7.
4. Conclusion
A series of ratiometric TP fluorescent Zn2+sensors containing quinoline and DPA moieties have been designed based on a newly synthesized probe. At first,five structures are designed by using the diazine,cyano and acyl groups to increase acceptor strength and the amino and dimethylamino terminals to enhance electron donating ability. The OPA and TPA properties of the experimental and designed probes before and after combination with Zn2+are computed at a DFT level. The calculated OPA and TPA wavelengths and TPA cross sections are consistent with the experimental trends. It shows that the OPA and TPA wavelengths is red-shifted and the TPA cross section is enhanced greatly because of the increased ICT mechanism upon metal binding. As expected,all the designed probes give rise to strong TPA responses at longer wavelengths. It is interesting to find that the addition of the acyl unit in the designed probe 4 is the most efficient way to increase TPA cross section due to the form of longer conjugated length and more linear backbone. It also reveals that one dimethylamino terminal attached along the skeleton can improve TPA intensity more efficiently than two side amino groups. Then, based on these results,we performed a second design using the most efficient acyl unit and dimethylamino terminal to increase both acceptor and donor strengths. The calculated TPA cross sections of the second designed probe 7 and its zinc complex 7Zn enhance significantly and the TPA wavelength of 7Zn exceeds 900 nm.At last,the emission properties of all the studied molecules are calculated by optimizing the first excited state. It exhibits that the designed probes 4 and 7 have large differences of emission wavelength between free ligand and zinc complex,which demonstrates that they are suitable for ratiometric detection.The fluorescence quantum yieldsφFof the designed probes 4 and 7 have also been calculated using the relation of the computed difference of dipole moment and the experimentalφF.It shows that theφFof the designed probes increase considerably. Our design is so successful that both the TPA cross section and the quantum yield are improved greatly. This work provides useful guidelines for the design of efficient ratiometric TP probes for zinc ions.
Acknowledgement
Project supported by the Shandong Provincial Natural Science Foundation,China(Grant No.ZR2020MA078).
- Chinese Physics B的其它文章
- A nonlocal Boussinesq equation: Multiple-soliton solutions and symmetry analysis
- Correlation and trust mechanism-based rumor propagation model in complex social networks
- Gauss quadrature based finite temperature Lanczos method
- Experimental realization of quantum controlled teleportation of arbitrary two-qubit state via a five-qubit entangled state
- Self-error-rejecting multipartite entanglement purification for electron systems assisted by quantum-dot spins in optical microcavities
- Pseudospin symmetric solutions of the Dirac equation with the modified Rosen–Morse potential using Nikiforov–Uvarov method and supersymmetric quantum mechanics approach

