Interpenetrating polymers supported on microporous polypropylene membranes for the transport of chromium ions☆
Yesid Tapiero,Julio Sánchez,Bernabé L.Rivas*
Polymer Department,Faculty of Chemistry,University of Concepción,Casilla 160-C,Concepción,Chile
1.Introduction
A current major environmental problem is water pollution.Because of fast industrial development,chromium,mercury,copper,nickel,cadmium,arsenic,and other water-contaminating species pollute our water systems.Chromium is a widely used heavy metal in many engineering and chemical industries because of its durability and aesthetic quality.
Stable ions,such as trivalent chromium Cr(III)and hexavalent chromium Cr(VI),are very common and present in different oxidation states in water and the environment.
In biological systems,Cr(VI)ions are water-soluble across all pH values and accumulates.Cr(III)ions are not as toxic as Cr(VI)ions in the environment.Cr(III)is an essentialelementfor life[1].Nevertheless,at certain concentrations,it can damage the biological permeability of cell membranes,ionic channels,receptors,and enzymes[1].These ions,especially Cr(VI),can cause serious health problems,such as cancer.This toxicity depends on the concentration and exposure period[2].The World Health Organization(WHO)recommends a maximum concentration limit of 0.05 mg·L-1[3].
Because Cr(VI)ions exist as oxyanions,their speciation depends on the pH and concentration.Dichromate(Cr2O72-),chromic acid(H2CrO4),and dichromate acid(H2Cr2O7)form if the pH is strongly acidic and the Cr(VI)ion is highly concentrated[4].This leads to a high quantity of chromate(CrO42-)ions at weakly acidic pH values above 6.0,causing this species to exist as an acid chromate(HCrO4-)and dichromate(Cr2O72-)ion mixture.
In industry,Cr(VI)and Cr(III)compounds are used as dye pigments,as well as in surface plating and chromium electro-plating(decorative and hardplating),aluminum anodizing,chemical industries,oxidative dyeing,cooling water towers,and so on,and there are more common sources for pollution.Chromium ions may also enter drinking water from corrosion inhibitors that are used in water pipes and containers[5].
The most common methods to remove Cr(VI)ions from polluted solutions are reduction and precipitation[6],absorption[7],and ion exchange[8].Cr(VI)has also been removed using membrane technologies.These technologies include reverse osmosis,nanofiltration,dialysis,ultra filtration,Donnan dialysis,membrane electrolysis,diffusion dialysis,and electrodialysis[9,10]using liquid,emulsified,and supported membranes[11-13].However,it is hard to remove ions when the ions are of the same valence and close in nature or size.In this particular case,Donnan dialysis stands out because it has applications in many areas,such as chemical analysis for pre-concentration,extraction processes,hydrometallurgy[14],separating acids from their salts[15],radioactive flow deacidification[16],removing copper and zinc using commercial cationic membranes[17],and removing inorganic anions,such as fluoride,nitrate,bromated,and borates,from drinking water[18-20].
Donnan dialysis uses chemical potential differences between membrane sides to generate ionic transport(the driving force)and maintain the electro-neutrality of the two solutions during the process[21].In addition,Donnan dialysis removes Cr(VI)ions using commercial anion exchange membranes,such as SB-6470,AFN,ACM,and Raipore 1030[14,22,23].
Selecting a suitable working membrane guarantees efficient Donnan dialysis processes.Therefore,functional membranes are ideally developed from chemically stable commercial porous materials.Some examples of commercial disposable micro-porous membranes are polypropylene(PP)or polyethylene.
Ion exchange pore- filled polymeric membranes with interpenetrating polymer networks(IPNs)are attractive candidates.These IPNs form when two or more polymer networks are partially and noncovalently crosslinked at the molecular scale.IPN architectures cannot be separated by link breakagesviachemical methods[8,24].
The disadvantage of ion exchange resins and membranes is their lack of selectivity in the presence of cation and anion mixtures in the pollution solution.Therefore,the development of selective membranes that may useN-methyl-D-glucamine(NMG)ligands would be interesting.NMG ligands have been added to water-soluble polymers and immobilized on resins to remove arsenic[25,26],chromium[27],and boron[28]ions from water.
The main goalofthis work is to modify PP membranes with selective ligand-ion exchange IPNs of poly(glycidyl methacrylate-NMG)P(GMANMG),as well as also complex networks,such as P(GMA-NMG)/P(AA),P(GMA-NMG)/P(AMPSA)and P(GMA-NMG)/P(ClVBTA).These membranes were studied for Cr(VI)ion transportviaDonnan dialysis.
2.Experimental
2.1.Materials
Macroporous(AN06 Merck Millipore,0.6-μm pore size)isotactic PP membranes were employed.Forthe activation ofthe PP membrane surface,the reagents employed were polyvinyl alcohol(PVA 15000,Merck),ethanol(Merck),and Type I deionized water from Thermofisher Scientific.The activation of the PP membrane surface method was described previously[26].
N-methyl-D-glucamine(NMG,Aldrich),2-acrylamido-2-methyl-1-propane sulfonic acid(AMPSA,Aldrich),ar-[(vinylbenzyl)trimethyl ammonium chloride] (ClVBTA, Aldrich),N,N′-methylene bisacrylamide(MBA,Aldrich),acrylic acid(AA,Aldrich),glycidyl methacrylate(GMA,Aldrich),and ammonium persulfate(APS,Merck)were employed to modify the polypropylene membrane for IPN synthesis.An aluminum flat-reactor was used for thermal radical polymerization of the functional IPNs.A stirred-cell filtration unit(Millipore,model 8050)was used to inject the reactive solution into the porous PP membrane.The synthesis of the glycidyl methacrylate-N-methyl-D-glucamine(GNMG)monomer is described in the literature[25].
A UB-10 pH/mV meter from Denver Instrument was used to measure the pH of the solutions,and HNO3(Merck)and NaOH(Merck)were used as controls for the pH.K2Cr2O7(Merck)was the chromium(VI)source,and NaCl(Merck)was the extraction reagent.
A Cary 100 Scan UV-Visible spectrophotometer from Varian was used to directly measure the Cr(VI)ion concentration[29,30].The Cr(VI)ion was measured at the wavelength 350 nm in a solution of pH 3.0 and at a wavelength of 370 nm in a solution of pH 9.0[29,30].Origin®Pro 8 software was used to build the transport profile.
2.2.Formation of IPNs inside the membranes
The functional monomers,crosslinking reagent(MBA)solution,and initiator agent(APS)in a 20-ml reaction solution were passed through the membrane[27].A stirred-cell filtration unit was used with a pressure of 0.1 MPa from nitrogen gas.Only one injection was performed.In situfree-radical polymerization was performed on the surface and inside the membrane pores at 70°C for 24 h,and APS at 1 mol%was employed as the radical-generating reagent[31].
Fig.1 shows the steps for the preparation of the modified membranes.The hydrophilic MPP and homopolymer network synthesis were obtained according to Table 1[26].
The experimentalmixture designs for IPNformation inside macropore polypropylene membranes were ofthree differenttypes.The firstmixture was 0.5 mol·L-1AA,0.2 mol·L-1GNMG,8 mol%MBA and 1 mol%APS.The second was a mixture of 0.5 mol·L-1AMPS,0.2 mol·L-1GNMG,8 mol%MBA and 1 mol%APS.The third was a mixture of 0.5 mol·L-1ClVBTA,0.2 mol·L-1GNMG,8 mol%MBA,and 1 mol%APS.
2.3.Characterization
2.3.1.Percentage of modification
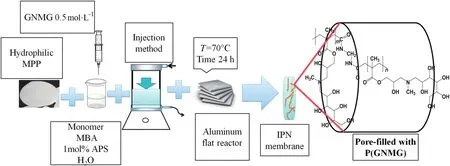
Fig.1.Illustrative scheme of preparation of the GNMG/functional IPNs.
The percentage of modification was gravimetrically measured.Initially,the dry,unmodified membranes were weighed.After the modification process,the dry-membrane samples were weighed again.The percent modification(ΔPM)was calculated from Eq.(1):

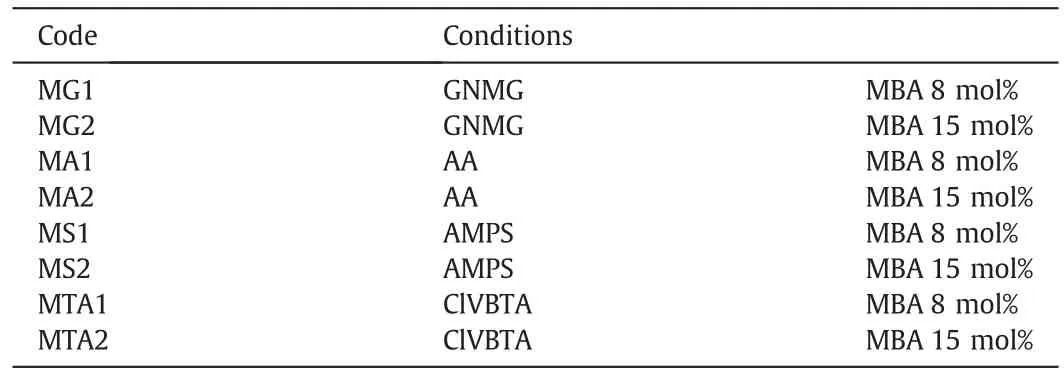
Table 1Experimental conditions for the synthesis ofthe interpenetrating homopolymer networks[26]
wherewfis the dry IPN-membrane mass(g)andw0is the unmodifiedmembrane mass(g).This method quantitatively calculated the mass percent of the ion exchange IPNs.
2.3.2.Water-uptake percentage
The mass ofthe IPN-modified dry membrane samples was measured before and after wetting with Type I deionized water for 24 h at room temperature.During this period,the samples reached their swelling equilibrium.The modified membranes are porous for this reason.The mass of these membranes was measured 3 times.In all of the tests,excessive water was removed from the modified wet membranes using absorbent paper.This method was gravimetric.The water-uptake percent(ΔWw)was calculated from Eq.(2):
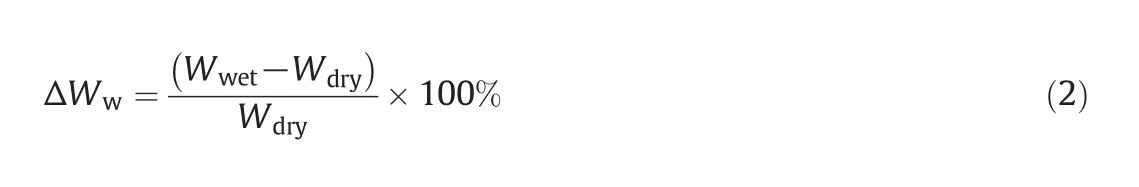
whereWwetis the wet IPN-membrane mass(g)andWdryis the dry IPN-membrane mass(g).
2.3.3.Volumetric permeateflux
The time required for 50 ml of deionized water to pass through the modified membrane was measured.The equipment used during this step was a stirred-cell filtration unit(Amicon)that works with nitrogen gas.This analysis probes the indirectformation ofthe IPNin the pores.A pressure of 0.1 MPa was maintained for all of the tests.
2.3.4.Scanning electron microscopy(SEM)
This technique was used to analyze the super ficial morphological changes in the modified and unmodified membranes.A 20,000-kV JOEL microscope(JSH 6380LV model)was used.
2.3.5.Fourier transform-infrared spectroscopy coupled to attenuated total reflectance(ATR-FTIR)
Using a Nicolet FT-IR instrument with a DTGS-KBr detector(Omnic 5.2 Nicolet Instrument Corp.),the FTIR spectra of the IPN-modified and unmodified membranes were obtained.The measurement was collected between 400 and 5000 cm-1.
2.3.6.Electrokinetic properties
The membrane samples were cut into thin pieces and submerged into 5 × 10-2mol·L-1KCl solutions at pH values of 3.0,7.0,and 9.0.Brookhaven ZetaPlus equipment was used.The values pH was controlled using HCl and NaOH.
The electrokinetic potential ϛ was determined from the ionic mobility(μe)by using the Smoluchowski(Eq.(3)):

where μeis the ionic electrophoretic mobility(cm2·V-1·s-1),ε is the liquid permittivity(J·V-2·m-1),and ϛ is the electrokinetic potential or the zeta potential(mV)[32,33].
2.3.7.Donnan dialysis analysis of the Cr(VI)ions
Fig.2 shows a dual-chamber cell working under the Donnan equilibrium principle,and the functional membrane was placed between the chambers.Each chamber had a 250 ml capacity and was filled with 150 ml of the working solution for the tests.
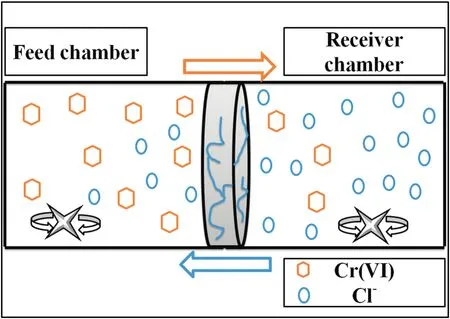
Fig.2.Scheme of the Donnan dialysis cell.
2.3.7.1.Experimentaldesign.Modified interpenetrating polymer network membranes were used for Cr(VI)ion transport.The feed chamber was filled with the Cr(VI)solution,and the extraction chamber was filled with a 1 mol·L-1NaCl solution as the extraction agent at pH 3.0 and 9.0.The Cr(VI)ion sources were 2.4 × 10-4mol·L-1and 1.2 × 10-4mol·L-1K2Cr2O7at pH 3.0 and 9.0,respectively.Every 60 min for 24 h,3 ml was extracted from the extraction chamber.
Direct UV-visible spectrophotometry was used to measure the Cr(VI)ion concentration[29].The samples were returned to the extraction chamber after reading the Cr(VI)ion concentration.The transport of Cr(VI)ions was evaluated based on the extraction percentage(E)calculated using Eq.(4):

whereCie,t,is the ion concentration in the extraction chamber,which changes with time.Cif,0is the ion concentration in the feed chamber at time of 0,andVeandVfare the extraction chamber and feed chamber volumes(L),respectively.
3.Results and Discussion
3.1.Morphological and microstructural characterization
The changes in the modification percent,water-absorption capacity,and volumetric flux must be considered for the evaluation of the modified membranes.These samples were compared with unmodified microporous membranes.
Fig.3a shows the change in the modified mass and water uptake percentage ofthe polyelectrolyte P(GMA-NMG)networks.The percentage ofmodification in allsamples gradually increased from0 to 7%atthe time the crosslink agent(MBA)concentration increased.However,the percentage mass modification value of the MG2 is lower than the ion exchange polyelectrolyte/NMG membranes.
The MG1 and MG2 membranes with the NMG single functional group achieved the lowest percentage of mass modification values,in comparison with the ion exchange polyelectrolyte/NMG membranes.The percentage ofwateruptake(see Fig.3a)for MG1and MG2 has a tendency to increase similarto the percentage mass modified values.These results imply that the porous MPP membranes are filled by crosslinked polyelectrolyte chains[34,35].The percentages of the water uptake values showed that the distribution of the functional NMG interpenetrating networks is not high or homogeneous inside the structure of the PP membrane.However,the highly hydrophobic nature of the PP does rejectthe aqueous reactive glycidylmethacrylate NMGmonomeric solution with a certain percentage.
Additionally,Fig.3a indicates that the polymeric networks composed of ion exchange polyelectrolyte and poly(NMG)achieved percentage mass modified values higher than the polymeric networks of the P(GNMG).The reason for this behavior is that the ion exchange functional groups(quaternary ammonium,sulfonate and carboxylic acid)of the monomers provided the NMG monomer complete access to the inside PP microporous membrane.The sulfonate monomer can enhance the aqueous reaction pressure impregnation with the NMG mixture(MSG membrane).The sulfonate monomer helps to reduce the super ficial tension between the aqueous reactive monomeric solution and the PP fiber surface in comparison with the acrylic acid and quaternary ammonium(MAG and MTAG membranes).The percentage of water uptake for MTAG,MAG,and MGS presents the same tendency to increase with the percentage mass modified values(see Fig.3a).The interpenetrating network concentration increases,and then,the water can introduce itself inside the structure membrane.
Fig.3b shows the change in the volumetric flux of the modified membranes in comparison with the unmodified PP membrane.The volumetric flux of water decreased for all modified membrane samples because the pore size decreased,which produced resistance to water because the interpenetrating networks can swell.Although MBA and ion exchange monomers provide a rigid structure for the membrane,it also contributes to their brittleness[36].However,a high degree of crosslinking is favorable for the stability of membranes[37].
Table 2 summarizes the percentage of modification,the percentage of water uptake,and the change in the volumetric flux of water by the interpenetrating homopolymer networks.These values are a function of the concentration of the crosslinking-reagent MBA.As the MBA concentration increased,the water uptake and hydrophilicity of the membranes decreased because of the compact structure of the IPNs.If the IPN concentration increases inside the membrane,then the volumetric flux of water would be lower.These differing results occurred because ofthe morphology in the layer shape and the agglomerated,amorphous particles[26,27,31].
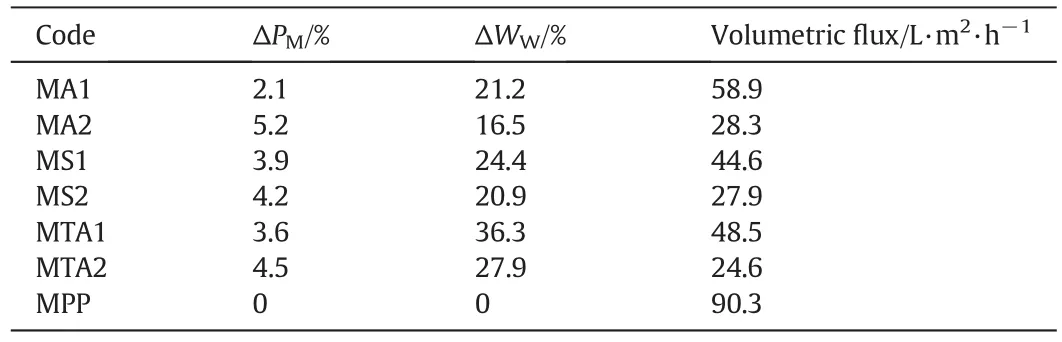
Table 2The values of the modified degree percentage,water uptake percentage and volumetric flux of the homogeneous modified membranes with interpenetrating homopolymer networks
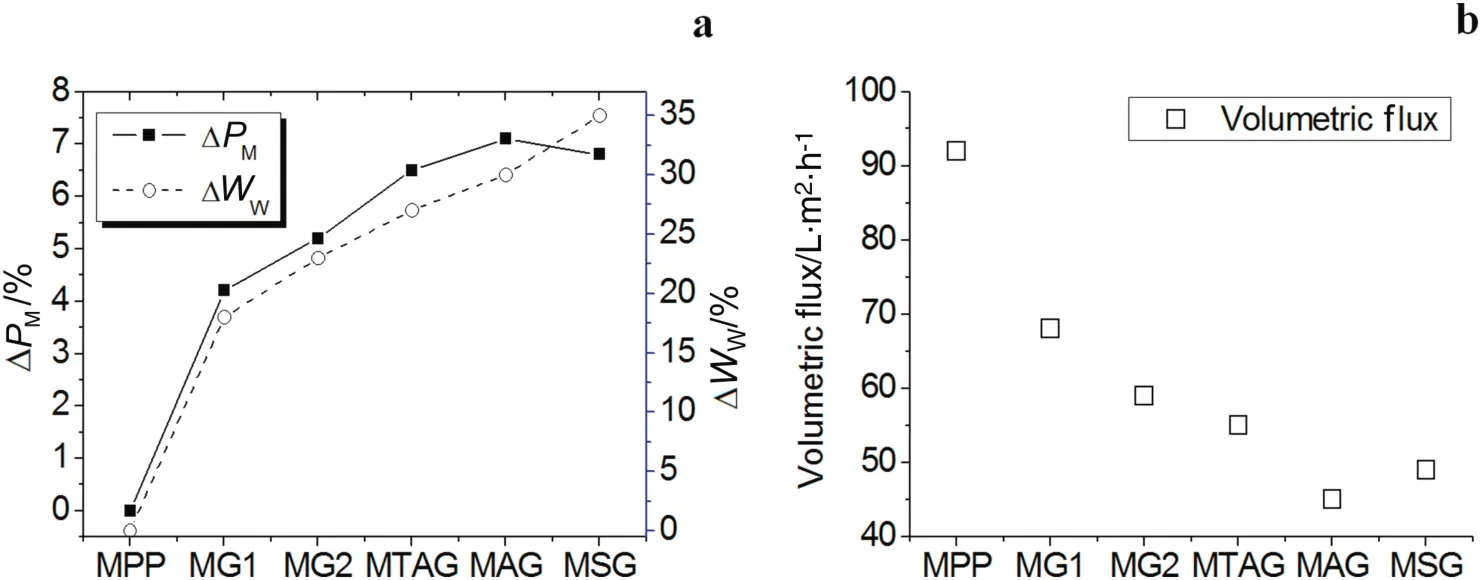
Fig.3.Percentage of modification in mass,percentage of water uptake,and volumetric flux of water of the modified IPNs membranes.
3.2.SEM analysis
The surface morphology of an unmodified macroporous MPP membrane is shown in Fig.4a and b.In Fig.4a,the membrane face exhibits low porosity,an intertwined fiber structure and a smooth super ficial aspect,while in Fig.4b,a high density was observed based on the thick,compacted fiber group.The membrane morphology changes for the IPN membranes were compared with those changes for the unmodified MPP membrane.
For example for the MG2,Fig.4c and d shows two phases of the P(GNMG)and PP,and these are relative to the unmodified MPP(see Fig.4a and b).These results represent an anisotropic membrane[38,39].The P(GNMG)networks were agglomerated in large quantities on the surface,which makes it easier to access the ion-exchange groups(see Fig.4d).However,in their cross-sections,the membrane achieved low IPN concentration because of the hydrophobic nature of the isotactic PP and low pore uniformity.Shindeetal.achieved stable modified PP membranes of the pore surface modification by grafting poly(glycidyl methacrylate)in the reaction with the NMG,and the SEM images are similar to those obtained in this research[5].
In the case of the MAG,on the top of the membrane,a high concentration of ion exchange domain particles(see Fig.4e)was present in comparison with the top ofthe MG2 membrane(see Fig.4c).This result is due to the poly(acrylic acid)fraction that improves the stability ofthe functional networks by hydrogen bonds between the carboxylic acid group and hydroxyl group of the NMG and the poly(vinyl alcohol)grafted on the PP fibers.However,in the cross-area,the quantity of P(AA-GNMG)was poor(see Fig.4f)because the neutral carboxylic acid did not help to improve the introduction of the initial reactive mixture inside the PP membrane.These domain particles are large and agglomerate[31].
Additionally,MSG membranes have small domain particles that are very homogeneous on the top of membrane in comparison with the MAG membrane(see Fig.4g).However,IPNs agglomerated in largequantities on the cross-section area(see Fig.4h).The P(AMPS-GNMG)networks tended to be more homogeneous and cover more polypropylene fibers than the P(AA-GNMG)networks.The sulfonate group makes accessing the ion-exchange groups easier inside the membrane.
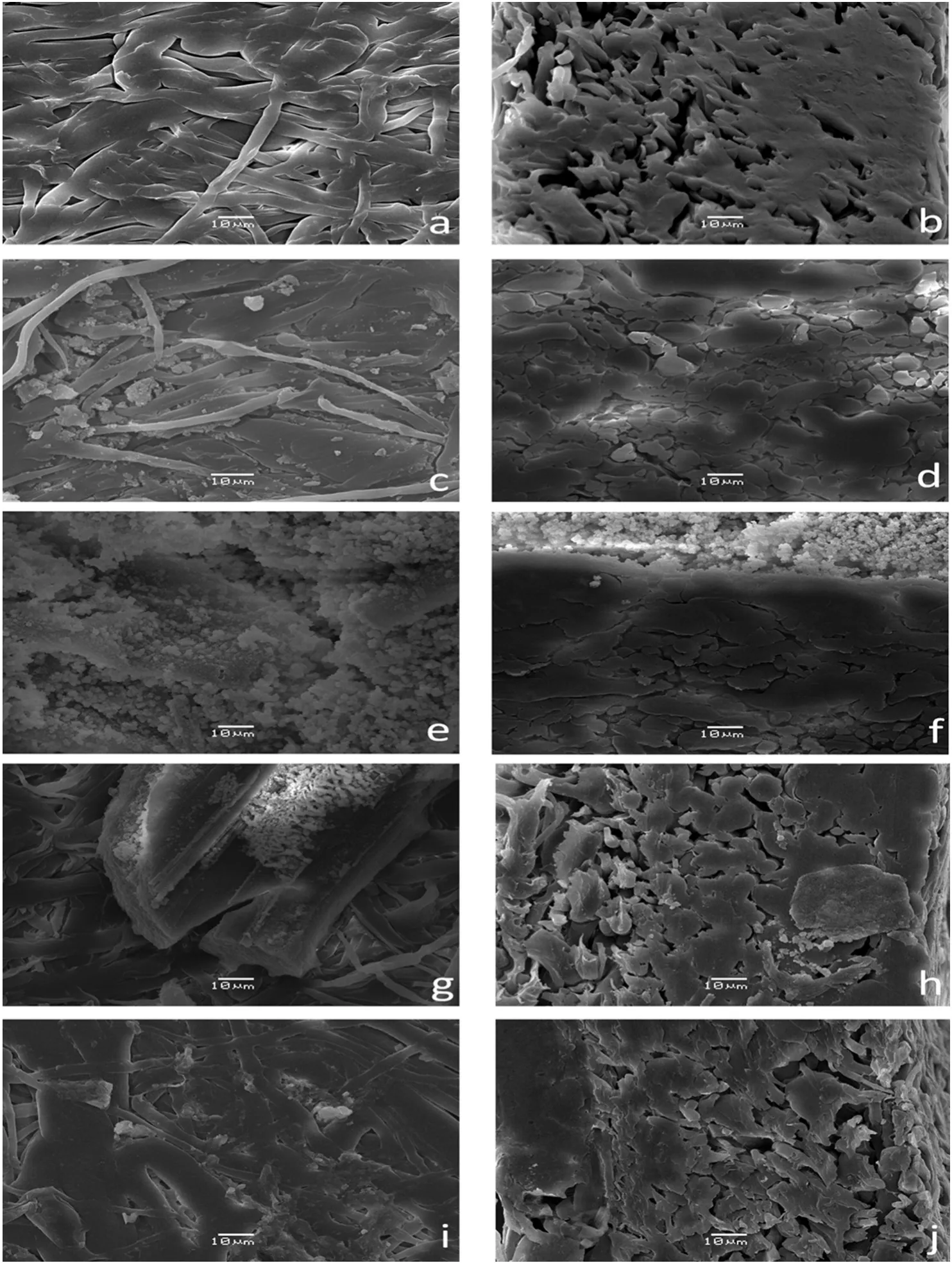
MTAG membranes achieved low formation of IPN domains on the top and the cross-section area in comparison with the other modified membranes(see Fig.4i,and j).This membrane exhibited low IPN concentrations in their cross-sections because of the hydrophobic nature ofthe isotactic PP and low pore uniformity because oftheirhighly asymmetric character[40,41].
Some research reports show similar results when a PP film was super ficially modified with sodium polystyrene sulfonate and a polyethylene film was modified with sodium polystyrene sulfonate.The main results found a structural surface change where small particles with small pores appeared[42].Similarly,poly(N,N-dimethylaminoethyl methacrylate)(PDMAEMA)was grafted and crosslinked onto the PP membrane surface.The membrane had 80%porosity with a 0.20-μm pore size[43].In the same way,MPP membranes had poly(vinylbenzyl ammoniumsalts)crosslinkedin situwithin its pores[44].Anotherstudy grafted and copolymerized a 4-vinylpyridinium monomer within PP membranes with 0.5-μm pores[45].
3.3.Fourier transform-infrared with total attenuated re flectance(ATR/FTIR)spectroscopy
In the microstructural analysis of the modified MPP membranes,the ATR/FT-IR absorption bands corresponding to the functional groups were determined.The unmodified MPP membrane had characteristic absorption bands at 2970-2800 cm-1corresponding to the stress and asymmetric stretching of C--H(δas)bonds in the(--CH3)methyl groups.The peak at 1480-1380 cm-1results from the C--H(δas)of the CH2bond flexion vibrations because these bonds have asymmetric deformations.The symmetric interaction flexion vibrations for the C--H(δs)in the CH3bonds were generated across the same range.The signals from 1300 to 700 cm-1represent the changing isotactic polypropylene microstructural characteristics.The signals at 1200,1116,998,841,and 800 cm-1are the isotactic con figuration crystallinity sequences[46](see Figs.5a and 6a).
研究组护理后的EPDS评分(5.16±1.21)分,常规组护理后的EPDS评分(10.83±1.58)分,组间对比差异具有统计学意义(t=-28.491 0,P=0.019 9,P<0.05)。
The signal at 970 cm-1corresponds to the monomeric head-to-tail isotactic PP con figuration[47].C--H and C--C bonds were observed in the spectra for the modified and unmodified MPP membranes.These signals are stronger in the modified membrane than in the unmodified MPP membrane.The absorption signals(ʋas(--CH2),δas(--CH2)and δs(--CH2))were stronger in the modified membrane samples than in the unmodified MPP membrane(see Figs.5a and 6a).
Fig.5b shows that the absorption band of the MAG at 1690 cm-1is due to the presence of a(C═O)stretching vibration.The band at 3400 cm-1is due to the(--OH)stretching vibration,overlapping bands at 1077 cm-1(C--O),1035 cm-1(C--N),one at 1174 cm-1(CH--OH),and 1563 cm-1(CO--O)[25].The absorption band at approximately 2930 cm-1is due to the C--H stretching of the polymer backbone,and the(C--O)bond stretching absorbed at 1150 cm-1.
Fig.5c shows that the absorption band of the MSG at 1644 cm-1is due to the stretching vibration of the(C═O)double bond and that 1453 cm-1is the vibration of(C--N).The bands at 1115 cm-1and 1047 cm-1are due to the asymmetric and symmetric vibrations of(S═O),respectively.The overlapping bands at 1077 cm-1correspond to(C--O).The band at 1035 cm-1corresponds to(C--N).The band at 1174 cm-1corresponds to(CH--OH),and the(C--O)stretching absorbed at 1150 cm-1and 1563 cm-1(CO--O)[25].
Fig.6b shows the absorption signals for MG1 with the NMG ligand group.The vibrational bands included overlapping bands at 1077 cm-1(C--O),1035 cm-1(C--N)and one at 1174 cm-1(CH--OH),and the vibrational bands included 1719 cm-1(C═O)and 1563 cm-1(CO--O)[25].
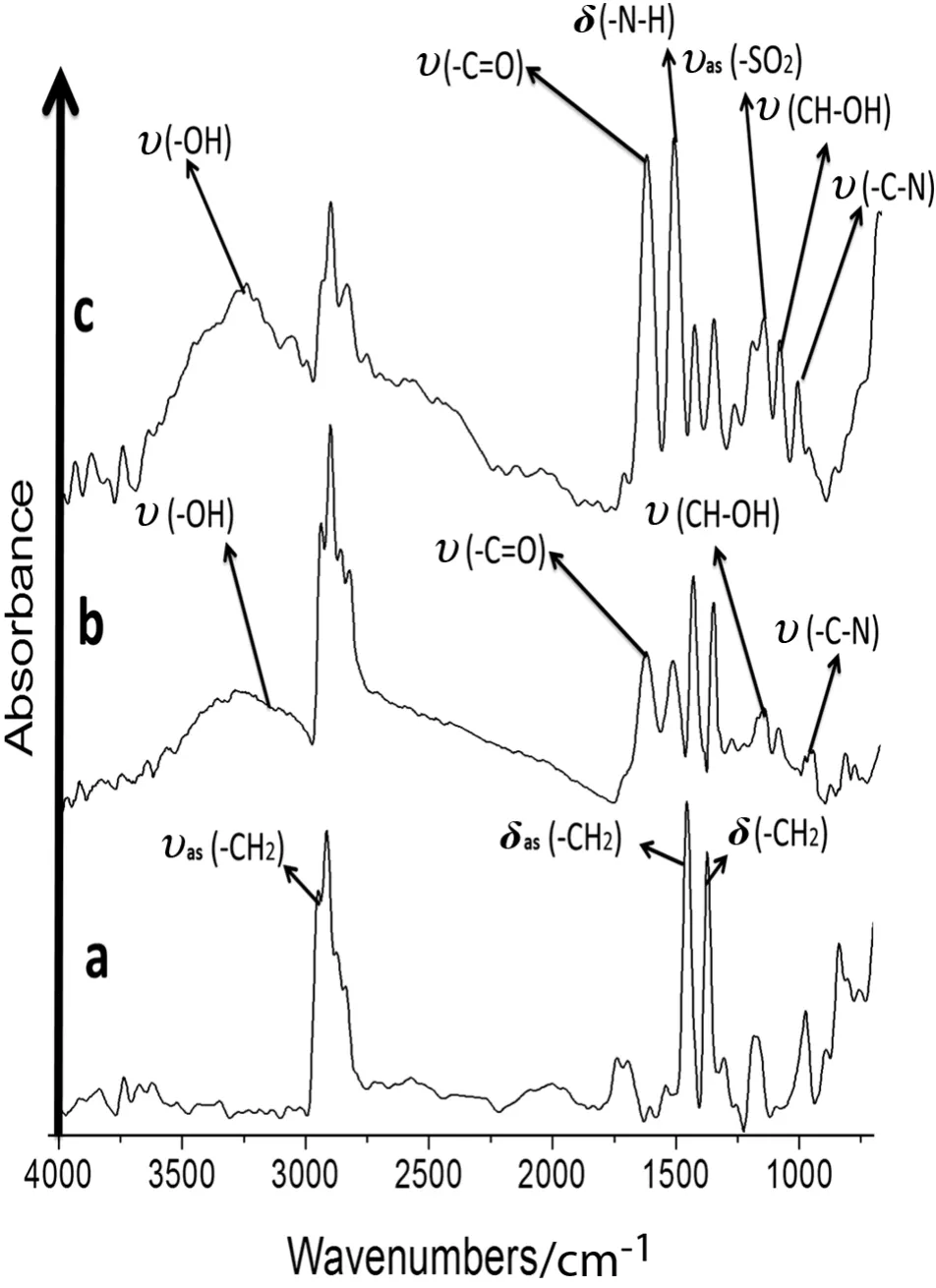
Fig.5.ATR/FT-IR of the anion-N-methyl-D-glucamine membranes and unmodified polypropylene.a.MPP.b.MAG.c.MSG.
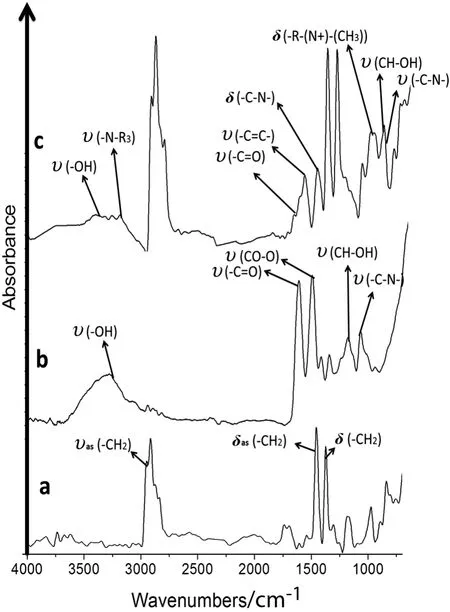
Fig.6.ATR/FT-IR of the cation-N-methyl-D-glucamine membranes and unmodified polypropylene.a.MPP.b.MG1.c.MTAG.
Fig.6c shows the absorptions band of MTAG with the aromatic(C═C)absorption signals occurring in the range from 1400 to 1600 cm-1,1077 cm-1(C--O),and 1035 cm-1(C--N),as well as one at 1174 cm-1(CH--OH).The vibrational bands included 1719 cm-1(C═O)and 1563 cm-1(CO--O)[25].The quaternary ammonium group has absorption signals at 1581 cm-1for the(N--H)bond and(C--N)vibration and at 1483 cm-1for the(--N+--(CH3))group bond flexion.
3.4.Electrokinetic properties
In the membranes,ionic functional groups cause super ficial charges.The surface charge is related to phenomena,such as wetting,adsorption,and ion transport.The electrokinetic potential is the fixed charge potential difference(cationic,anionic,weak acid and chelating groups)between the mobile ionic charges in the absorption layer[26].
The electrokinetic potential depends on the IPN concentration,pH,and capacity of the electric double layer formed.
Fig.7,Curve a shows the behavior of MTAG,which exhibited higher electrokinetic potentials when the pH was 3.0.Because the NMG and quaternary ammonium groups have a positive charge,the network was swollen.The excess H+repels the quaternary ammonium and NMG groups with positive charges.However,at a pH of 9.0,the electrokinetic potential decreases because of the presence of OH-ions.The network was contracted,and these OH-ions compete with the Cl-ions in the diffusion layer.The movement of the Cl-counterion in the diffusion layer becomes favored when the pH is 3.0.
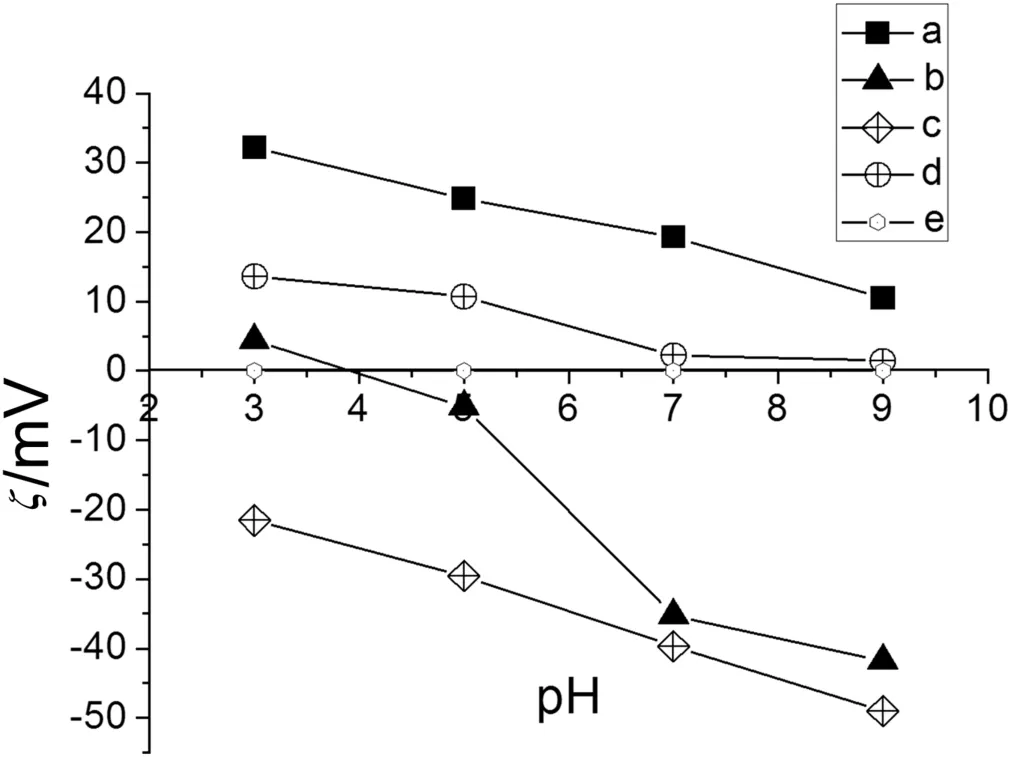
Fig.7.Electrokinetic potential of modified membranes with IPNs with 5 × 10-2 mol·L-1 of KCl.a.MTAG.b.MAG.c.MSG.d.MG2.e.MPP.
MSG exhibited the most negative electrokinetic potential values over the pH range tested.The sulfonate groups can overlap the positive charge of NMG at acidic pHs(see Fig.7,Curve c).When the pH was 3.0,the electrokinetic potential became less negative because H+ions competed with Na+ions in the diffusion layer.These results depended on the acid character of the functionalized surface,and the excess OH-ions repelled the groups fixed in the network and favored the movement of the Na+counterion in the diffusion layer.
In Fig.7,Curve d shows the behavior ofMG2.Atlow pHvalues,ithas a positively charged structure,butathigh pHvalues,itis uncharged.The MPP membrane is hydrophobic without any modification and has no ionic functional groups,which renders the membrane inert to electrical fields(see Fig.7,Curve e).
3.5.Donnan dialysis analysis of the Cr(VI)ions
The modified pore- filled PP membranes with IPNs were evaluated to determine the Cr(VI)ion transport capacity.Four conditions for the Cr(VI)ions were used,an acid medium(pH 3.0)and basic medium(pH 9.0),each at two different concentrations(1.2 × 10-4mol·L-1and 2.4 × 10-4mol·L-1),in the food chamber.For all of the tests,the extraction reagent was 1 mol·L-1NaCl.
For the concentration of 1.2 × 10-4mol·L-1of Cr(VI),Fig.8a shows the Cr(VI)ion extraction percentage profile of the modified pore- filled membranes with pH 3.0 compared with wetted unmodified PP membrane with ethanol.TheEvalues were highest for this probe,35.7%of the MTA1,32.7%of the MTAG and 26.9%of the MG2.TheEvalues were highest for the concentration 2.4 × 10-4mol·L-1of Cr(VI)at pH 3.0(see Fig.8b),and they were 63.3%of the MTAG,56.7%of the MG1,and 46.1%of the MG2.
TheEvalues were high for the high concentration of Cr(VI)than the low concentration of Cr(VI)because of the saturation of the functional groups.All modified membranes achieved Donnan equilibrium between 250 min and 300 min at pH 3.0.The values ofEfor the modified membranes are shown in Table 3.
At pH 3.0,the modified membranes with ammonium quaternary and NMG groups achieved swollen networks because of the excess of electrostatic repulsion forces.These electrostatic forces were generated between the nitrogens with a positive charge.However,the passive transport of Cr(VI)ions to the extraction chamber occurred because of the concentration gradient.However,the NMG group acts as a ligand and ion exchange functional group at pH 3.0.In the same way,a strong quaternary ammonium group interaction with HCrO4-and Cr2O72-ions exists,decreasing the ion exchange velocity and increase the ion exchange relationship[49].The Cr(VI)ionic fluxes depend on the morphological changes of the modified membranes with respect to that of MPP[4].
The MTA1 membrane showed the best results in all of the tests.The object of this study was to compare composite(P(GMA-NMG)/Polyelectrolyte)interpenetrating polymer network membranes with homo-polyelectrolyte interpenetrating polymer network membranes,and it was found that the extraction percentage values of the Cr(VI)ions are very close to the values of the homo-polyelectrolyte membranes.
At pH 3.0,the poly(acrylic acid)networks were protonated and the networks produced an hydrophobic effect.This reason can explain the lowEvalues.However,in the MAG,the NMG groups participated in ion exchange and chelating effects for the Cr(VI)ions.However,the concentration gradient can exceed to the retention effect of the membrane,but with a low efficiency.
The modified membraneswith sulfonate groupspresentan excessof negative charge in the pore walls and in the membrane surface.The repelling interaction between the Cr(VI)ions and negative surface is known as the Donnan repulsion principle.This principle limits the force of the concentration gradient between the two chambers.Therefore,theEvalues are low for the MS1,MS2 and MSG.The participation of the NMG group in this process is only for retention because the positive charge is neutralized.This whole process occurs under acidic conditions.
Fig.8c shows the results that were obtained from the Cr(VI)ions extraction percentage using a low concentration of 1.2 × 10-4mol·L-1in the food chamber at pH 9.0.TheEvalues highest for this probe were 56.7%of the MTA1,48.5%of the MTAG and 32.0%of the MG1.The test using the highest concentration of 2.4 × 10-4mol·L-1of Cr(VI)achieved 65.2%of the MTA1,60.5%of the MTAG,and 47.5%of the MG1(see Fig.8d).This behavior is very similar to that obtained for the high concentration of 2.4 × 10-4mol·L-1of the Cr(VI)in the food chamber test at pH 3.0.The values ofEfor the modified membranes are shown in Table 3.
Moreover,the evaluation of the Cr(VI)ion transport through the modified membranes at pH 9.0 was more efficient compared to the transport obtained at pH 3.0.The forces between the Cr(VI)ions and the quaternary ammonium were overcome by the concentration gradient force for the diffusion passes through the MTA1,MTA2 and MTAG.
At pH 9.0,the NMG does not have a positive charge because the nitrogen is not protonated.For this reason,the polymeric chains of the functional networks generate a hydrophobic effect,but the glucoside function produces a chelating effect.MG2 achieves a retention effect for the low and high concentration ofCr(VI)because ofthe crosslinking,while the concentration gradient forces are highest for the MG1.
AtpH 9.0,the Donnan exclusion principle is highestfor the sulfonate groups of the MS1,MS2 and MSG.These membranes achieve a strong excess of negative charge that excludes the CrO42-ions.This is the reason for the low transport.It is possible that the membrane presents locations at which ion leakage can occur to the extraction chamber.In the same way,MA1,MA2 and MAG with carboxylic acid groups are produced from the Donnan exclusion principle at pH 9.0.The carboxylic acid groups are ionized completely,and a strong excess of the negative charge excludes the CrO42-ions.
The main reason for the exclusion was due to the change in the ionic conduction properties of the functional groups,and this situation helped to develop the concentration gradient.For example,at pH 9.0,Donnan equilibrium was achieved between 150 min and 250 min;in comparison with the test at pH 3.0.At pH 9.0,most Cr(VI)ions are in the CrO42-form.Cr(VI)ions compete against the hydroxyl ions in the ion exchange inside the membrane.Additionally,the size difference of the CrO42-,HCrO4-and Cr2O72-ions produces friction to the movement and a strong electrostatic force in the ion exchange.
4.Conclusions
The IPNs can be prepared using microporous PP membranes with ion exchange-ligands through radical polymerization.The concentrations of the glycidyl methacrylate NMG monomer,the crosslinked agent MBA and the proper ion exchange monomer play an important role in membranes with IPNin situsynthesis because they can control the density of the formed networks.The modified MPPs were valued in terms of their water uptake,modified mass,morphology,and transport capacities.The pore- filled polypropylene membranes,including P(GMA-NMG),and the complex networks,including P(GMA-NMG)/P(AA),P(GMA-NMG)/P(AMPSA),and P(GMA-NMG)/P(ClVBTA)IPNs,were evaluated using SEM,FT-IR analysis,and their electrokinetic properties.The modified membranes were compared with the unmodified PP membranes.Through these results,it was demonstrated that IPNs formed inside the pores.
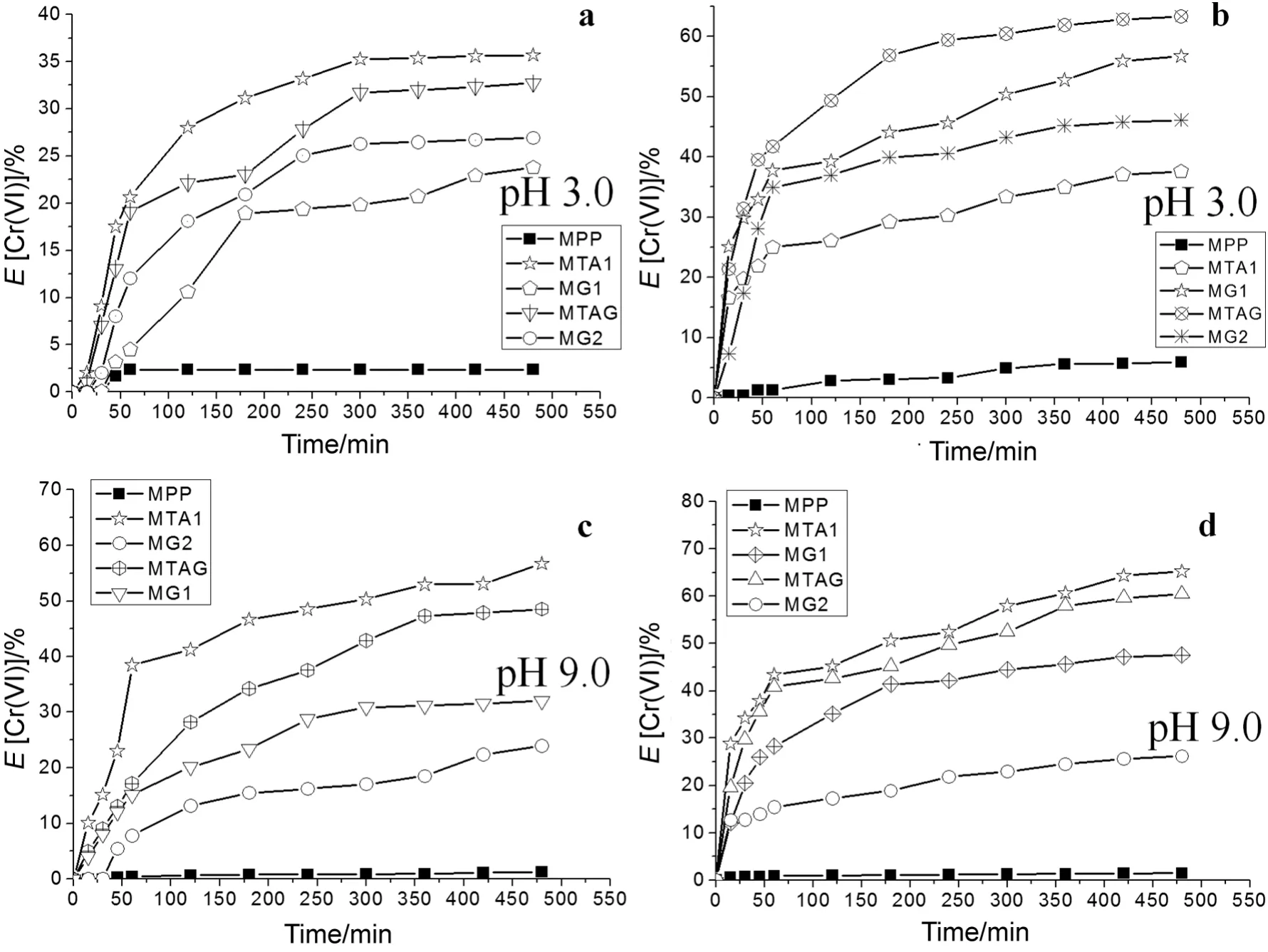
Fig.8.Extraction percentage of Cr(VI)ions.a.Low concentration of Cr(VI)(1.2 × 10-4 mol·L-1)at pH 3.0.b.High concentration of Cr(VI)(2.4 × 10-4 mol·L-1)at pH 3.0.c.Low concentration of Cr(VI)(1.2 × 10-4 mol·L-1)at pH 9.0.d.High concentration of Cr(VI)(2.4 × 10-4 mol·L-1)at pH 9.0.
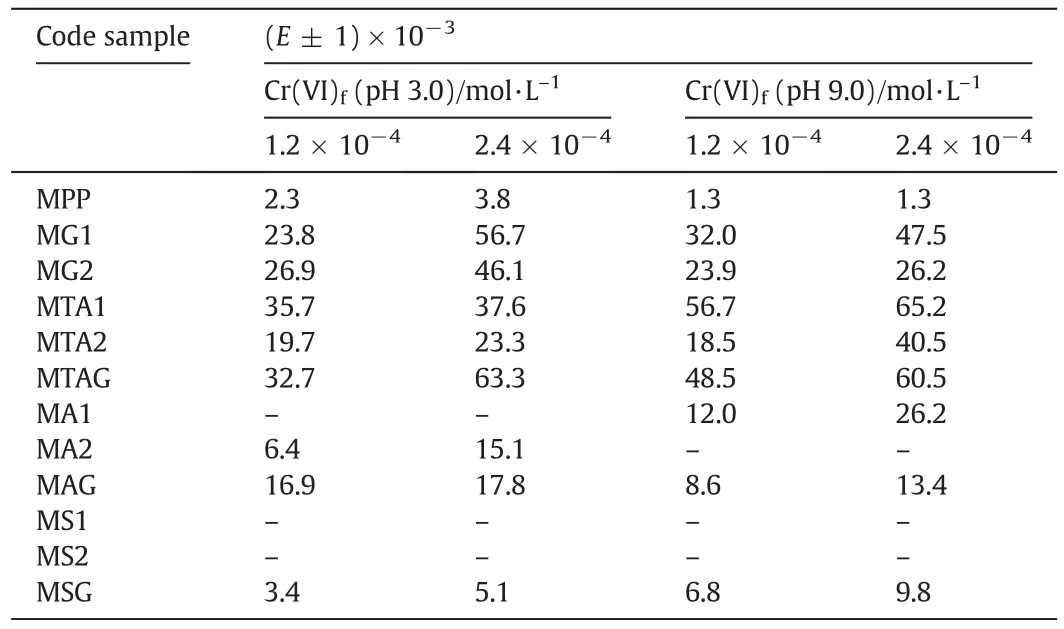
Table 3The values of the extraction percentage(E)in the receiving chamber
The transport properties under the Donnan dialysis principle for the Cr(VI)ionswere evaluated as a function ofthe concentration in the feed(low and high)and the pH values.From the obtained results,the MTA1 membrane was chosen because ofthe obtained results(35.7%)from the use ofCr(VI)ion transportata pH of3.0 and a low food concentration of Cr(VI).However,MTAG is used because its Cr(VI)ions have a transport capacity of 63.3%at pH 9.0 and a high food concentration of Cr(VI).
At pH 9.0,MTA1 achieved 56.7%of the extraction of the Cr(VI)ions when a low concentration of Cr(VI)was used.At a high concentration of Cr(VI),this membrane achieved 65.2%of the extraction of the Cr(VI)ions.In both cases,with an acidic or basic pH,the MTAG membrane achieved results of the Cr(VI)extraction very close to the MTA1.
These results were in fluenced by various factors,including the super ficial charges of the membranes,solution concentration of the feed phase(low and high),and steric effects.The Donnan exclusion principle and equilibrium in fluenced the ion transport.
[1]G.-R.R.Bernardo,R.-M.J.Rene,A.-D.l.T.Ma Catalina,Chromium(III)uptake by agrowaste biosorbents:Chemical characterization,sorption-desorption studies,and mechanism,J.Hazard.Mater.170(2009)845-854.
[2]M.Owlad,M.K.Aroua,W.A.W.Daud,S.Baroutian,Removal of hexavalent chromium-contaminated water and wastewater:A review,Water Air Soil Pollut.200(2009)59-77.
[3]WHO,Guidelines for Drinking Water Quality,World Health Organization,Genova,2008.
[4]T.Sardohan,E.Kir,A.Gulec,Y.Cengeloglu,Removal of Cr(III)and Cr(VI)through the plasma modified and unmodified ion-exchange membranes,Sep.Purif.Technol.74(2010)14-20.
[5]S.Kalidhasan,A.S.K.Kumar,V.Rajesh,N.Rajesh,The journey traversed in the remediation of hexavalent chromium and the road ahead toward greener alternatives—A perspective,Coord.Chem.Rev.317(2016)157-166.
[6]R.R.Patterson,S.Fendorf,M.Fendorf,Reduction of hexavalent chromium by amorphous iron sul fide,Environ.Sci.Technol.31(1997)2039-2044.
[7]D.Park,S.-R.Lim,Y.-S.Yun,J.M.Park,Reliable evidences that the removal mechanism of hexavalent chromium by natural biomaterials is adsorption-coupled reduction,Chemosphere70(2007)298-305.
[8]W.Zheng,J.Hu,Z.Han,E.Diesel,Z.Wang,Z.Zheng,C.Ba,J.Langer,J.Economy,Interactions of Cr(VI)with hybrid anion exchange/porous carbon fibers in aqueous solution at natural pH,Chem.Eng.J.287(2016)54-61.
[9]F.Melak,G.Du Laing,A.Ambelu,E.Alemayehu,Application of freeze desalination for chromium(VI)removal from water,Desalination377(2016)23-27.
[10]M.Bodzek,Application of membrane techniques for the removal of micropollutants from water and wastewater,Copernican Lett.6(2015)24-33.
[11]S.Saravanan,K.M.S.Begum,N.Anantharaman,Removal of hexavalent chromium by emulsion liquid membrane technique,J.Univ.Chem.Technol.Metall.41(2006)333-342.
[12]P.Venkateswaran,K.Palanivelu,Studies on recovery of hexavalent chromium from plating wastewater by supported liquid membrane using tri-n-butyl phosphate as carrier,Hydrometallurgy78(2005)107-115.
[13]A.Bhowal,S.Datta,Studies on transport mechanism of Cr(VI)extraction from an acidic solution using liquid surfactant membranes,J.Membr.Sci.188(2001)1-8.
[14]A.Tor,Y.Çengeloğlu,M.Ersöv,G.Arslan,Transport of chromium through cationexchange membranes by Donnan dialysis in the presence of some metals of different valences,Desalination170(2004)151-159.
[15]S.Koter,M.Kultys,B.Gilewicz-Łukasik,I.Koter,Modeling the transport of sulfuric acid and its sulfates(MgSO4,ZnSO4,Na2SO4)through an anion-exchange membrane,Desalination342(2014)75-84.
[16]J.Mathur,M.Murali,M.B.Krishna,V.Ramachandhran,M.Hanra,B.Misra,Diffusion dialysis aided electrodialysis process for concentration of radionuclides in acid medium,J.Radioanal.Nucl.Chem.232(1998)237-240.
[17]A.A.Said,M.Amara,H.Kerdjoudj,The effect of thiourea as a complexing agent on the separation of metallic ions through cation exchange membranes by Donnan dialysis,Ionics19(2013)177-183.
[18]Y.S.Dzyazko,S.L.Vasilyuk,L.M.Rozhdestvenskaya,V.N.Belyakov,N.V.Stefanyak,N.Kabay,M.Yüksel,Ö.Arar,Ü.Yüksel,Electro-deionization of Cr(VI)-Containing Solution.Part II:Chromium transport through inorganic ion-exchanger and composite ceramic membrane,Chem.Eng.Commun.196(2008)22-38.
[19]S.Nataraj,K.Hosamani,T.Aminabhavi,Potential application of an electrodialysis pilot plant containing ion-exchange membranes in chromium removal,Desalination217(2007)181-190.
[20]A.Narębska,M.Staniszewski,Separation of carboxylic acids from carboxylates by diffusion dialysis,Sep.Sci.Technol.43(2008)490-501.
[21]E.H.Cwirko,R.G.Carbonell,A theoretical analysis of Donnan dialysis across charged porous membranes,J.Membr.Sci.48(1990)155-179.
[22]E.Castillo,M.Granados,J.L.Cortina,Chemically facilitated chromium(VI)transport throughout an anion-exchange membrane:Application to an optical sensor for chromium(VI)monitoring,J.Chromatogr.A963(2002)205-211.
[23]E.Castillo,M.Granados,J.L.Cortina,Chromium(VI)transport through the Raipore 1030 anion exchange membrane,Anal.Chim.Acta464(2002)15-23.
[24]P.Thapliyal,Interpenetrating polymer networks,Compos.Interface17(2010)85-89.
[25]L.Toledo,B.L.Rivas,B.F.Urbano,J.Sánchez,NovelN-methyl-D-glucamine-based water-soluble polymer and its potential application in the removal of arsenic,Sep.Purif.Technol.103(2013)1-7.
[26]Y.Tapiero,J.Sánchez,B.L.Rivas,Ion-selective interpenetrating polymer networks supported inside polypropylene microporous membranes for the removal of chromium ions from aqueous media,Polym.Bull.1-25(2015).
[27]Y.Tapiero,B.L.Rivas,J.Sanchez,Functional ion membranes supported inside microporous polypropylene membranes to transport chromium ions:Determination of mass transport coefficient,J.Chil.Chem.Soc.59(2014)2737-2746.
[28]J.Sánchez,J.Wolska,E.Yörükoğlu,B.L.Rivas,M.Bryjak,N.Kabay,Removal of boron from water through soluble polymer based onN-methyl-D-glucamine and regenerated-cellulose membrane,Desalin.Water Treat.57(2016)861-869.
[29]O.Thomas,C.Burgess,UV-visible Spectrophotometry of Water and Wastewater,Elsevier,2007.
[30]R.Burke,R.Mavrodineanu,Acidic potassium dichromate solutions as ultraviolet absorbance standards,Standardization in Spectrophotometry and Luminescence Measurements:Proceedings of a Workshop Seminar Held at the National Bureau of Standards,Gaithersburg,Maryland,November,November 19-20,1975,US Department of Commerce,National Bureau of Standards 1976,p.121.
[31]Y.Tapiero,B.L.Rivas,J.Sánchez,M.Bryjak,N.Kabay,Polypropylene membranes modified with interpenetrating polymer networks for the removal of chromium ions,J.Appl.Polym.Sci.132(2015).
[32]A.Szymczyk,P.Fievet,J.Reggiani,J.Pagetti,Electrokinetic characterization of mixed alumina-titania-silica MF membranes by streaming potential measurements,Desalination115(1998)129-134.
[33]J.Zhou,X.Zhang,Y.Wang,X.Hu,A.Larbot,M.Persin,Electrokinetic characterization of the Al2O3ceramic MF membrane by streaming potential measurements,Desalination235(2009)102-109.
[34]S.-J.Seo,B.-C.Kim,K.-W.Sung,J.Shim,J.-D.Jeon,K.-H.Shin,S.-H.Shin,S.-H.Yun,J.-Y.Lee,S.-H.Moon,Electrochemical properties of pore- filled anion exchange membranes and their ionic transport phenomena for vanadium redox flow battery applications,J.Membr.Sci.428(2013)17-23.
[35]R.-Q.Fu,J.-J.Woo,S.-J.Seo,J.-S.Lee,S.-H.Moon,Covalent organic/inorganic hybrid proton-conductive membrane with semi-interpenetrating polymer network:Preparation and characterizations,J.Power Sources179(2008)458-466.
[36]J.Sun Koo,N.-S.Kwak,T.S.Hwang,Synthesis and properties of an anion-exchange membrane based on vinylbenzyl chloride-styrene-ethyl methacrylate copolymers,J.Membr.Sci.423(2012)293-301.
[37]R.Liu,L.Wu,J.Pan,C.Jiang,T.Xu,Diffusion dialysis membranes with semiinterpenetrating network for alkali recovery,J.Membr.Sci.451(2014)18-23.
[38]M.Ulbricht,Advanced functional polymer membranes,Polymer47(2006)2217-2262.
[39]H.Matsuyama,M.Yuasa,Y.Kitamura,M.Teramoto,D.R.Lloyd,Structure control of anisotropic and asymmetric polypropylene membrane prepared by thermally induced phase separation,J.Membr.Sci.179(2000)91-100.
[40]Q.Yang,Z.-K.Xu,Z.-W.Dai,J.-L.Wang,M.Ulbricht,Surface modification of polypropylene microporous membranes with a novel glycopolymer,Chem.Mater.17(2005)3050-3058.
[41]J.Schauer,J.Hnát,L.Brožová,J.Žitka,K.Bouzek,Heterogeneous anion-selective membranes:In fluence of a water-soluble component in the membrane on the morphology and ionic conductivity,J.Membr.Sci.401(2012)83-88.
[42]I.S.M.T.Maddanimath,S.R.Sainkar,K.Vijayamohanan,K.I.Shaikh,Patil,S.P.Vernekar,Humidity sensing properties of surface functionalized polyethylene and polypropylene films,Sensors Actuators B81(2002)141-151.
[43]Y.-F.Yang,L.-S.Wan,Z.-K.Xu,Surface hydrophilization for polypropylene microporous membranes:A facile interfacialcrosslinking approach,J.Membr.Sci.326(2009)372-381.
[44]A.K.Pandey,A.Goswami,D.Sen,S.Mazumder,R.F.Childs,Formation and characterization of highly crosslinked anion-exchange membranes,J.Membr.Sci.217(2003)117-130.
[45]C.-c.Wang,F.-l.Yang,L.-F.Liu,Z.-m.Fu,Y.Xue,Hydrophilic and antibacterial properties of polyvinyl alcohol/4-vinylpyridine graft polymer modified polypropylene non-woven fabric membranes,J.Membr.Sci.345(2009)223-232.
[46]D.Y.Xinyuan Zhu,Hongxi Yao,Pingfang Zhu,In situ FTIR spectroscopic study of the regularity bands and partial-order melts of isotactic poly(propylene),Macromol.Rapid Commun.21(2000)354-357.
[47]E.Pretsch,P.Bühlmann,C.Affolter,E.Pretsch,P.Bhuhlmann,C.Affolter,Structure Determination of Organic Compounds,Springer,2000.
[48]Y.Qiu,K.Park,Environment-sensitive hydrogels for drug delivery,Adv.Drug Deliv.Rev.64(2012)49-60.
[49]T.Sata,Ion Exchange Membranes:Preparation,Characterization,Modification and Application,Royal Society of Chemistry,UK,2004.
 Chinese Journal of Chemical Engineering2017年7期
Chinese Journal of Chemical Engineering2017年7期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
- Implications from protein uptake kinetics onto dextran-grafted Sepharose FF coupled with ion exchange and affinity ligands☆
- Effects of solubility parameter differences among PEG,PVP and CA on the preparation of ultra filtration membranes:Impacts of solvents and additives on morphology,permeability and fouling performances
- Experimental detection of bubble-wall interactions in a vertical gas-liquid flow☆
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
