Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
Yang Luo, Liang Deng, Qiu-Ju Deng, Li WenDepartment of Gastroenterology, the First Affiliated Hospital of Chongqing Medical University, Chongqing 40006, ChinaDepartment of Genetics, Beijing Cancer Hospital, Beijing 004, ChinaDepartment of Cardiology, First Affiliated Hospital of Chongqing Medical University, Chongqing 40006, China
Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
Yang Luo1*, Liang Deng1, Qiu-Ju Deng2, Li Wen31Department of Gastroenterology, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
2Department of Genetics, Beijing Cancer Hospital, Beijing 100142, China
3Department of Cardiology, First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
ARTICLE INFO ABSTRACT
Article history:
in revised form 16 March 2016 Accepted 15 April 2016
Available online 20 June 2016
Chitooligosaccharides Gastric cancer
Radiotherapy
radiosensitization
Cell cycle
Apoptosis
Objective: To observe the chitooligosaccharides (COS) effect on the proliferation inhibition and radiosensitivity of three types of human gastric cancer cell line. Mothods: CCK-8 assay was employed to obtain the inhibition ratio of COS on BGC823 cells, MKN45 cells and SGC7901 cells at 48 h after treatment and the proliferation-inhibition curve was drawn with the inhibition ratio of COS on three types of cells. The clonogenic assay was used to detect the cell viability of 0, 1, 2, 4, 6 and 8 Gy (6 dose grades) in RAY group and RAY+COS group after X-ray, and the cell survival curve was used to analyze the sensitization enhancement ratio of COS. Flow cytometry was employed to detect cell cycle and apoptosis rate in control group, RAY group and RAY+COS group after 48 h treatment. Results: COS inhibited the proliferation of three types of cells. The inhibition rate was positively correlated with the concentration of COS, and the susceptibility of MKN45 cells, SGC7901 cells and BGC823 cells to COS decreased in turn. The cell viability decreased gradually with the increasing radiation dose in RAY group and RAY+COS group (P<0.01). The cell viabilities of RAY+COS group were lower than those of RAY group at all the dose grades under X-ray exposure(P<0.01), and the sensitization enhancement ratios of COS on BGC823 cells, MKN45 cells and SGC7901 cells were 1.06, 1.28 and 1.15 respectively. In controlled trials, apoptosis rate and percentage in the G2/M phase of three types of cells in RAY+COS group were higher than those in control group and RAY group, and percentage in the S phase and the G0/G1phase in RAY+COS group were lower than those in the other two groups (P<0.01). Conclusions: COS can inhibit the proliferation of three types of human gastric cancer cells and enhance the radiosensitivity by inducing apoptosis and G2/M phase arrest.
1. Introduction
With the progress of medical science and technology, the incidence and mortality of gastric cancer show a trend of decline gradually in the worldwide, but China is still a high-risk area where the cases of newly-increased and death in each year are more than 40% and 35% of total amount of the world[1]. A survey data of cancer epidemiology of China in 2010 showed that standardized incidence and mortality of gastric cancer all ranked third in malignant tumor,23.71% and 16.64% respectively[2,3], and it’s of great significance to actively explore the effective treatment for many patients with gastric cancer. Although surgical resection is still the preferred way to radically cure gastric cancer currently, the recurrence and mortality of a single surgery are more than 80% in 5 years after operation. Based on this background, radiotherapy as an irreplaceable and important supplementary method has been used in each stage of clinical treatment comprehensively[4,5]. Althoughradiotherapy shows more and more advantages in the treatment and research of gastric cancer, damage of radioactive rays on healthy tissue still can’t be ignored. The use of excellent radiosensitizer is effective measure to lower the side-effect of radiotherapy. Chitooligosaccharides (COS) is one of the concerned hot-topics of biological medical workers in recent years and past research has found that it not only possesses the good effect like oxidation reaction and activation of autoimmune, but also has positive effect to inhibit tumor development[6,7]. However, the use of COS synergistic radiation is still seldom reported so far. This research selects three types of human gastric cancer cell line including BGC823, MKN45 and SGC7901 as objective to conduct an experiment to compare COS effect on their proliferation inhibition and radiosensitization. Moreover, we preliminary explore the mechanism of action and hope to provide theoretical basis for seeking more efficient and harmfulless treatment scheme for gastric cancer. Presently reports are the following.
2. Materials and methods
2.1. Cell lines, experimental materials and experimental methods
A total of 6 concentration levels of COS were established and each level was conducted simultaneously with 3 holes in parallel samples. Three cell clines of continuous cell culture to logarithmic phase (human gastric cancer including BGC823, MKN45, SGC7901 cells, Shanghai Institute of Cells, Chinese Academy of Sciences)were diluted as 4×104/L of concentration through digestion and joined into 96-well plates according to 0.1 mL/well, and then were placed in a suitable environment for adherent growth (CO2incubator,Shanghai Gemtop Scientific Instrument Co., LTD). The fresh culture medium (0.11 mL/well) diluted with COS (Shanghai HuiCheng Biotechnology Co., LTD) was replaced after 24 h and 0, 1.0, 2.0,3.0, 4.0, 5.0 mg/mL of concentrations respectively were added into COS in each well. After infiltrating 48 h, CCK-8 reagent was added into COS along the well (0.01 mL/well) (CCK-8 kit, Shanghai LiRui biological technology co., LTD), and then the reagent and culture solution were mixed by tapping culture plate. All levels of absorbance (OD) were detected at λ=450 nm in 4 h after reacting fully. The experiment was repeated for 3 times to investigate the proliferation suppression effect of COS on three types of cells, which is the basis to select 1.0 mg/mL of COS concentration to conduct a follow-up study.
A total of 6 dose levels of X-ray were established, and each level was divided into RAY group and RAY+COS group and each group was conducted simultaneously with 3 holes in parallel samples. Different concentrations of single-cell suspension were joined into 6 wells culture-plate according to the inoculation amount of 0 Gy dose level (200/well), 1, 2 Gy dose level (500/well), 4, 6 Gy dose level (2 000/well) and 8 Gy dose level (5 000/well), and were placed in a suitable environment for adherent growth. Appropriate COS(1.0 mg/mL) was added into each well of RAY+COS group after 6 h and equivalent quantity of culture medium was added into each well of RAY group after infiltrating for 48 h. Tissue analogue with about 1 cm thickness was affixed on the culture plates in two groups and was conducted with X-ray irradiation in a distance of 100 cm and dosage rate of 2 Gy/min. (electron linear accelerator, Nanjing Chuang Rui Ying Biological Technology Co., LTD). Culturing for 12 d continuously, the number of cell mass consisted over 50 U was calculated through washing, fixing and dyeing. The experiment was repeated for 3 times to calculate data and draw up the cell survival curve, and sensitization enhancement ratio was calculated by the value of final slope (D0): SER=D0(RAY)/D0(RAY+COS).
Three groups were established and conducted simultaneously with 3 holes in parallel samples. The cell suspension of 1×105/L concentration was inoculated into 6-well plates and was placed in a suitable environment for adherent growth for 24 h. Appropriate COS (1.0 mg/mL) was added into RAY+COS group and equivalent quantity of culture medium was added into non-treatment group and RAY group. RAY group and RAY+COS group were infiltrated for 48 h, and then received 6 GyX-ray irradiation. Cultivating 48 h after replacing fresh medium, cell cycle and apoptosis rate in all groups were detected through the processing of digestion, wash and dilution.
2.2. Statistical methods
SPSS19.0 statistical software was used to perform statistical analysis. The distribution ratio of OD value, survival rate, apoptosis rate and cell cycle were all expressed as mean±SD. ANOVA was used to analyze the OD value of various COS concentration levels and cell survival rate of X-ray dose levels among many groups. T-test was used to compare the cell survival rate under all X-ray doses of RAY group and RAY+COS group, and cell cycle and apoptosis rate in the third group of control group. P<0.05 showed statistical significance.
3. Results
3.1. Anti-proliferative effect of COS on BGC823 cells,MKN45 cells and SGC7901 cells
OD values of BGC823 cells, MKN45 cells and SGC7901 cells under COS concentrations (0 mg/mL) were used as reference standard. The cell survival rate of three types of cells was decreased with a higher concentration of infiltrating after disposing various COS concentrations for 48 h and the difference was statistically significant (P<0.01). The COS was positively correlated with growth inhibition ratio and the concentration of treatment of three types of cells. The cell survival rate of three types of cells also showedsignificant difference among groups after treatment with COS of various concentration levels. COS showed the stronger suppression effect on MKN45 followed by SGC7901 and showed the minimum effect on BGC823. The difference of 1.0 mg/mL COS concentration level comparison was statistically significant (P<0.05). OD values comparison in three groups has significant statistical difference(P<0.01) when COS concentration increased to 2.0, 3.0, 4.0 and 5.0 mg/mL (Table 1).

Table 1Anti-proliferative effect of COS on three types of cells.
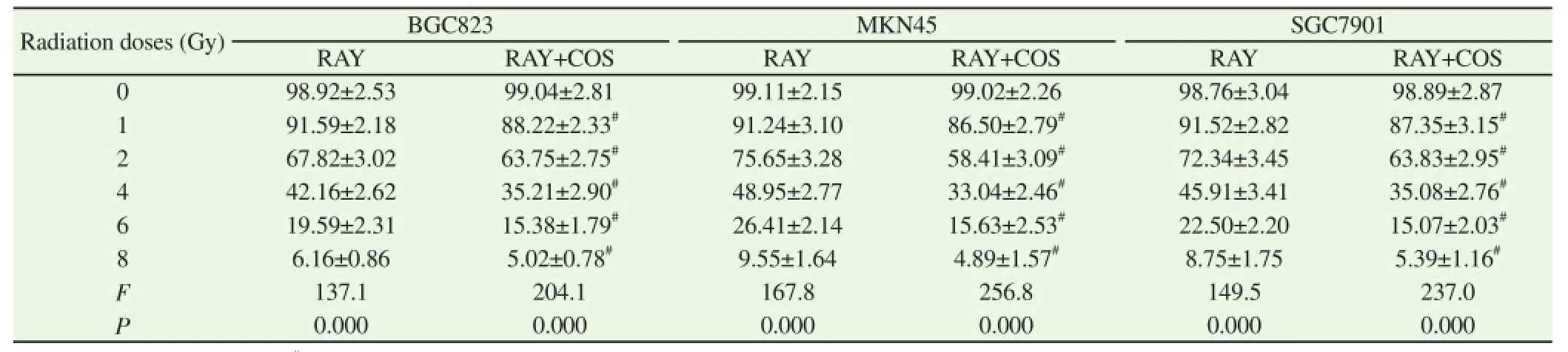
Table 2Comparison of cell survival rate of three types of cells under different radiation doses in RAY group and RAY+COS group.
3.2. Comparison of the cell survival rate of different dose levels of irradiation among groups
Survival rates of BGC823 cells, MKN45 cells and SGC7901 cells under 0 Gy dose of irradiation were used as reference standard. The survival rate of three types of cells in RAY group and RAY+COS group was decreased with enlargement of dose of irradiation and the difference was statistically significant (P<0.01) and the amplitude of RAY+COS group was greater. The cell survival rate of RAY+COS group was significant lower as the doses were 1, 2, 4, 6 and 8 Gy and the difference was statistically significant (P<0.01). The sensitization enhancement ratios of COS on BGC823 cells, MKN45 cells and SGC7901 cells were 1.06, 1.28 and 1.15 respectively obtained by drawing radiation survival curve of three types of cells (Table 2).
3.3. Comparison of apoptosis rate between groups
Observing three types of cells respectively, the apoptosis rates of RAY group and RAY+COS group were all more stimulating and increasing than non-treatment group, which had significant difference (P<0.01) and the apoptosis rate of RAY+COS group increased significantly than that in RAY group, which had significant difference (P<0.01) (Table 3 and Figure 1).
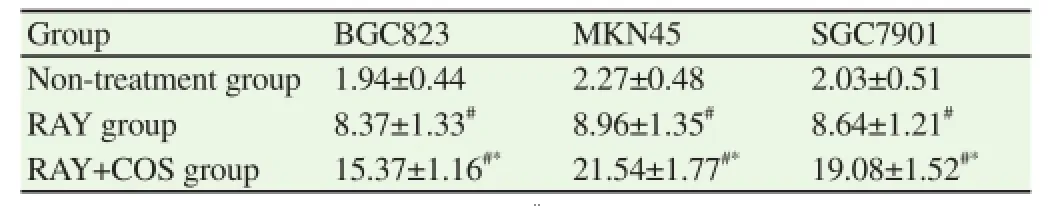
Table 3Comparison of apoptosis rate of three types of cells in each group (%).

Figure 1. Comparison of cell apoptosis condition of three types of cells in each group.
3.4. Comparison of cell cycle distributions between groups
Observing three types of cells respectively, G2/M phase proportion of RAY group and RAY+COS group was significant elevated than non-treatment group and the percentage of S phase and G0/G1phase was reduced, which was significant difference (P<0.01). The percentages of S phase and G0/G1phase of RAY+COS group were smaller and the duration of G2/M phase was prolonged significantly,which was significant difference (P<0.01) (Tables 4).

Table 4Comparison of cell cycle distribution of BGC823, MKN45 and SGC7901 cells in three groups (%).
4. Discussion
Modern unhealthy eating habit and onerous pressure of life are all the high risk factors to cause frequent gastric cancer. The morbidity and mortality of gastric cancer has reached 29.9/100 000 and 22.3/100 000 respectively in 2010 year of our country and the number of death has been accounted for 23.3% of the death toll due to the cancer[8,9]. Therefore, it is urgent and meaningful to seek efficient and feasible drugs and treatment methods against gastric cancer.
COS is the outstanding outcome of development of sugar biology and its all aspects like water solubility, absorptivity and affinity of human body have a unique advantage on high molecular sugars. Hence, COS is the one of foci of investigators on biological medicine. The research has verified the anti-tumor effect of COS on the leucocythemia, breast cancer and bladder cancer and scholars believed that its mechanism involved in multiple approaches[7,10,11]. In this research, COS also revealed the general proliferation inhibitory effect on human gastric cancer cells (BGC823, MKN45 and SGC7901) and the growth inhibiting rate of three types of cells was positively correlated with COS concentration. At the same time, we also found that the three types of cells showed sensitivity difference against the effect of growth retardation of COS (MKN45 is the strongest, followed by SGC7901 and BGC823) and the different outcome still needs further study.
Previous theory showed that gastric cancer cells have a strong resistance on radioactive rays, while the surrounding organs are easily suffered collateral damage. Hence, radiotherapy is not recommended to use in therapeutic schedule of gastric cancer[12]. However, with the rapidly development of positioning technology of computer and widespread use of radiosensitizer, radiotherapy nowadays has already occupied irreplaceable important position in the treatment of gastric cancer, which was used as assisted surgery to achieve the aim of reducing difficulty in the removal and the local recurrence, and prolonging the survival time[13,14]. For enhancing the effect of radiotherapy and protecting healthy tissue escaped from collateral damage of radioactive rays simultaneously, researchers are always seeking the efficient and reliable radiosensitizer. In this research, the survival rates of three types of human gastric cancer cells in RAY group and RAY+COS group were sharply reduced with the increasing of radiological dose. The combined application of COS and X-ray significantly elevated the killing effect. The COS promoted radiosensitivity of BGC823 cells, MKN45 cells and SGC7901 cells (1.06, 1.28 and 1.15, respectively)
Further control experiment showed that the synergistic effect of radiotherapy of COS on the three types of cells was correlated with surging of apoptosis rate. The apoptosis rates of three types of cells(BGC823, MKN45 and SGC7901) in RAY+COS group were more elevated than RAY group (1.84, 2.21 and 2.40). Apoptosis is an autologous dominant order-maintenance mechanism of organism to maintain its steady state operation and to realize the selfelimination of old, weak and sick cells and to save resources and earn a more favourable survival condition for organism. Apoptosis is one of the critical pathways in inducing normal cell death. It is inseparable between unlimited breeding cycle of cancer cells and regulating the disorder of autologous apoptosis process. The study of Tan and Mates et al. respectively reported that COS involved by different approaches can promote the apoptosis of cancer cells, such as disrupting physiological potential difference of mitochondrial membrane, releasing cytC into endochylema or reducing the environment of GSH activity and stimulating the peroxidation damage[15,16], which speculate that COS possess the gain effect on the apoptosis of cancer cells induced by radioactive rays.
Nowadays, the cell cycle sub-distribution is one of the heated topics of radiation biology research. The results of this research showed that COS can stimulate the deviation of cycle phase distribution of three types of human gastric cancer cells at the same time. The proportion of G2/M phase was significantly increased and the proportions of G0/ G1phase and S phase were relatively lower. G2/M phase showed the strongest response on irradiation injury, while G1phase and S phase will greatly reduce the duration of DNA damage and repair[17,18].The change of cell cycle distribution will significantly reduce the defense capability of cancer cells against X-ray and is helpful to improve the efficacy of radiotherapy. The operation of cell cycle process is according to a series of perfect and rigorous procedural evolution. Nowadays, the known regulating and control mediums are mainly including three major types (cyclin, CDK and CKI), which transform as the interaction and common manipulation of all factors,while the acting site of COS proliferation cycle distribution is still unknown[19,20].
In conclusion, COS possesses general growth retardation and radiosensitizing effect on three types of human gastric cancer and SER of MKN45, SGC7901and BGC823 cells were 1.06, 1.28 and 1.15 . The combined application of COS and radiotherapy can induce the increase sharply of apoptosis rate of cancer cells and the deviation of cycle phase distribution. Further research on anti-tumor effect of COS is conducted to open a window of hope for many patients with gastric cancer.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, et al. Annual report on status of cancer in China, 2010. Chin J of Cancer Res 2014; 26(1): 48.
[2] Fock K. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther 2014; 40(3): 250-260.
[3] Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol 2013; 107(3): 230-236.
[4] Boda-Heggemann J, Weiss C, Schneider V, Hofheinz R-D, Haneder S,Michaely H, et al. Adjuvant IMRT/XELOX radiochemotherapy improves long-term overall-and disease-free survival in advanced gastric cancer. Strahlenther Onkol 2013; 189(5): 417-423.
[5] Dikken JL, Baser RE, Gonen M, Kattan MW, Shah MA, Verheij M, et al. Conditional probability of survival nomogram for 1-, 2-, and 3-year survivors after an R0resection for gastric cancer. Ann Surg Oncol 2013;20(5): 1623-1630.
[6] Xu W, Jiang C, Kong X, Liang Y, Rong M, Liu W. Chitooligosaccharides and N-acetyl-D-glucosamine stimulate peripheral blood mononuclear cell-mediated antitumor immune responses. Mol Med Rep 2012; 6(2): 385-390.
[7] Kim E-K, Je JY, Lee SJ, Kim YS, Hwang JW, Sung SH, et al. Chitooligosaccharides induce apoptosis in human myeloid leukemia HL-60 cells. Bioorg Med Chem Lett 2012; 22(19): 6136-6138.
[8] Guo P, Huang Z, Yu P, Li K. Trends in cancer mortality in China: an update. Ann Oncol 2012; 23(10): 2755-2762.
[9] Li WQ, Ma JL, Zhang L, Brown L M, Li JY, Shen L, et al. Effects of Helicobacter pylori treatment on gastric cancer incidence and mortality in subgroups. J Natl Cancer Inst 2014; 106(7): dju116.
[10] Fernandes JC, Sereno J, Garrido P, Parada B, Cunha MF, Reis F, et al. Inhibition of bladder tumor growth by chitooligosaccharides in an experimental carcinogenesis model. Mar Drugs 2012; 10(12): 2661-2675.
[11] Rezakhani L, Rashidi Z, Mirzapur P, Khazaei M. Antiproliferatory effects of crab shell extract on breast cancer cell line (MCF7). J Breast Cancer 2014; 17(3): 219-225.
[12] Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol 2014; 20(7): 1635.
[13] Chang JS, Lim JS, Noh SH, Hyung WJ, An JY, Lee YC, et al. Patterns of regional recurrence after curative D2 resection for stage Ⅲ (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol 2012; 104(3): 367-373.
[14] Pang X, Wei W, Leng W, Chen Q, Xia H, Chen L, et al. Radiotherapy for gastric cancer: a systematic review and meta-analysis. Tumor Biol 2014;35(1): 387-396.
[15] Luo Z, Dong X, Ke Q, Duan Q, Shen L. Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation. Oncol Rep 2014; 32(5): 2215-2222.
[16] Jeong HW, Cho SY, Kim S, Shin ES, Kim JM, Song MJ, et al. Chitooligosaccharide induces mitochondrial biogenesis and increases exercise endurance through the activation of Sirt1 and AMPK in rats. PloS One 2012; 7(7): 40073.
[17] Dillon M, Good J, Harrington K. Selective targeting of the G2/M cell cycle checkpoint to improve the therapeutic index of radiotherapy. Clin Oncol 2014; 26(5): 257-265.
[18] Alexander BM, Pinnell N, Wen PY, D’Andrea A. Targeting DNA repair and the cell cycle in glioblastoma. J Neuro Oncol 2012; 107(3): 463-477.
[19] Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 2013; 140(15): 3079-3093.
[20] Uhlmann S, Mannsperger H, Zhang JD, Horvat EÁ, Schmidt C,Kublbeck M, et al. Global microRNA level regulation of EGFR-driven cell-cycle protein network in breast cancer. Mol Syst Biol 2012; 8(1): 570.
Document heading 10.1016/j.apjtm.2016.04.014
15 February 2016
*Corresponding author: Yang Luo, Department of Gastroenterology, the First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China
Tel: 13220283858
E-mail: luoyang_88victory@126.com
Foundation project: This research is supported by Youth Science Fund Project (No. 81400612).
 Asian Pacific Journal of Tropical Medicine2016年6期
Asian Pacific Journal of Tropical Medicine2016年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
- Study on prevention effect of Zishen Yutai pill combined with progesterone for threatened abortion in rats
- Correlation study of biological characteristics of non-small cell lung cancer A549 cells after transfecting plasmid by microbubble ultrasound contrast agent
- Expression and significance of angiostatin, vascular endothelial growth factor and matrix metalloproteinase-9 in brain tissue of diabetic rats with ischemia reperfusion
- Change of the peripheral blood immune pattern and its correlation with prognosis in patients with liver cancer treated by sorafenib
- Are efforts up to the mark? A cirrhotic state and knowledge about HCV prevalence in general population of Pakistan
